Dyslipidemia is common in people with diabetes and CKD. The risk of CVD is greatly increased in this population. People with diabetes and CKD should be treated according to current guidelines for high-risk groups.
4.1 Target LDL-C in people with diabetes and CKD stages 1-4 should be < 100 mg/dL; <70 mg/dL is a therapeutic option. (B)
4.2 People with diabetes, CKD stages 1-4, and LDL-C ≥ 100 mg/dL should be treated with a statin. (B)
4.3 Treatment with a statin should not be initiated in patients with type 2 diabetes on maintenance hemodialysis who do not have a specific cardiovascular indication for treatment. (A)
Diabetes is associated with a high risk of morbidity and premature mortality.433,434 Most adverse diabetes outcomes are due to macrovascular or microvascular complications.134 Macrovascular complications are common and severe; up to 80% of patients with type 2 diabetes will develop or die of CVD. Based on this severity of risk, prevention and management of CVD must be considered in the care of patients with diabetes and CKD. Therefore, these patients are strong candidates for treatment of dyslipidemia. Modifying CVD risk by using lipid-lowering agents is of tremendous importance and is a cost-effective strategy in people with type 2 diabetes.435,436
The NKF-KDOQI™ CPGs for Managing Dyslipidemia in CKD were established recently and CPGs for CVD in Dialysis Patients added new information on the inverse association between cholesterol level and mortality.6,10 The purpose of this guideline is to focus on patients with diabetes and CKD and to update the previous guidelines. Results from the first prospective randomized trial in hemodialysis patients with diabetes and indirect evidence from a recent post hoc analysis of 3 large multicenter trials on the beneficial effects of lipid-lowering therapy in diabetes and CKD were added to this guideline.
For this guideline, we included studies of patients with type 1 or type 2 diabetes and CKD stages 1 to 5. Because of the high prevalence of diabetes in the population, many individuals with other types of CKD also may have diabetes. In general, the guidelines for use of lipid-lowering agents in CKD stages 1 to 4 due to diabetes and other causes agree with each other,174-177although there is no direct or indirect evidence for treating patients with CKD stage 4. Because CKD per se markedly increases the risk of CVD, CKD may be considered a risk equivalent for CVD when assessing the need for lipid lowering.
Most patients with diabetes and CKD have dyslipidemia. (Strong)
Patients with diabetes and CKD typically have low levels of high-density lipoprotein cholesterol (HDL-C), high triglyceride levels, and average levels of LDL-C; LDL particles in people with diabetes tend to be smaller, denser, and possibly more atherogenic.437-440
Elevated LDL-C can effectively be treated with statins in diabetes and CKD. (Strong)
In general, cholesterol lowering with statin therapy is efficacious in patients with diabetes, including those without manifest coronary heart disease and those with relatively low LDL-C levels.97,98
Most patients with diabetes and CKD are at very high risk to develop macrovascular complications. (Strong)
The Adult Treatment Panel III (ATP III) guidelines of the National Cholesterol Education Program (NCEP)175 identified diabetes as a high-risk condition. This designation was based on evidence that most patients with diabetes have a greatly increased 10-year risk (>20%) of developing CVD. The onset of CVD in patients with diabetes carries a poor prognosis, both at the time of an acute CVD event and in the postevent period. In the Heart Protection Study (HPS), patients who had both diabetes and CVD were at very high risk of future CVD events.97 When type 2 diabetes is complicated by CKD, the cardiovascular risk increases dramatically; in particular, in patients with microalbuminuria or macroalbuminuria, it is approximately 2 to 4 times as high as in normoalbuminuric patients.40 The presence of CKD can be considered an additional cardiovascular risk factor per se.

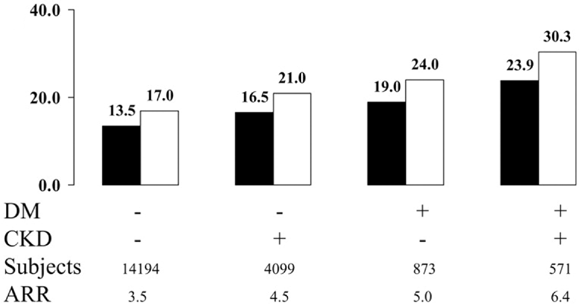
Figure 19. Effect of Pravastatin on the Absolute Risk Reduction (ARR) of Fatal Coronary Disease, Nonfatal Myocardial Infarction, or Coronary Revascularization by CKD and Diabetes (DM) Status. Reprinted with permission.99
LDL-C–lowering therapy decreases the risk of CVD in diabetes and CKD stages 1 to 3. (Moderate)
Primary and secondary prevention trials, including those in people with diabetes, have documented substantial cardiovascular benefit from administration of statins.441, 442 The recent primary prevention Collaborative Atorvastatin Diabetes Study (CARDS) reported an impressive decrease in cardiovascular deaths in people with type 2 diabetes in the absence of markedly decreased kidney function.98 In terms of absolute risk reduction, patients in the HPS with diabetes and CVD received the greatest benefit from statin therapy.97
A post hoc analysis of data from the PPP (a subject-level database combining results from 3 randomized trials of pravastatin, 40 mg daily, versus placebo) included 19,737 subjects, of whom 4,099 (20.8%) had CKD, but not diabetes, at baseline; 873 (4.4%) had diabetes, but not CKD; and 571 (2.9%) had both conditions.99 CKD was defined as eGFR less than 60 mL/min/1.73 m2 or GFR of 60 to 89.9 mL/min/1.73 m2 with trace or more proteinuria. The primary composite outcome was time to myocardial infarction, coronary death, or percutaneous/surgical coronary revascularization. The incidence of the primary outcome was lowest in individuals with neither CKD nor diabetes (15.2%), intermediate in subjects with only CKD (18.6%) or only diabetes (21.3%), and highest in subjects with both characteristics (27.0%). Pravastatin significantly reduced the risk of the primary outcome by 25% in subjects with CKD and concomitant diabetes and by 24% in subjects with neither characteristic. The absolute reduction in risk of the primary outcome due to pravastatin use was highest in subjects with both CKD and diabetes(6.4%) and lowest in subjects with neither characteristic (3.5%; Fig 19). This study provides indirect evidence that pravastatin treatment effectively decreases the risk of CVD in diabetes with CKD stages 1 to 3. The study does not provide evidence for a protective effect of pravastatin in more advanced stages of CKD because patients with more severe impairment of kidney function were excluded from the trials. More advanced stages of CKD also were excluded from the West of Scotland Coronary Prevention Study (WOSCOP),443 a primary prevention trial, and from the Cholesterol and Recurrent Events (CARE) Study444 and the Long-term Intervention with Pravastatin in Ischemic Disease (LIPID) Study,445 2 secondary intervention studies.
In the opinion of the Work Group, people with diabetes and CKD (other than stage 5) should receive LDL-C–lowering therapy. The high risk associated with diabetes and CKD supports initiation of statin therapy when LDL-C is greater than 100 mg/dL, with an option to achieve an LDL-C goal of less than 70 mg/dL.
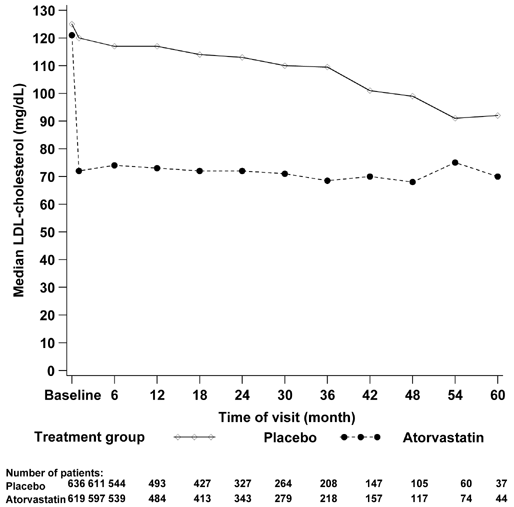
Figure 20. Median Change in LDL-C Concentrations From Baseline Until the End of the 4D.
To convert values for LDL-C to mmol/L, multiply
by 0.02586. Reprinted with permission.100
Atorvastatin treatment in patients with type 2 diabetes on maintenance hemodialysis treatment does not improve cardiovascular outcomes. (Strong)
The 4D, a multicenter, randomized, double-blind, and prospective study, randomly assigned 1,255 patients with type 2 diabetes on maintenance hemodialysis to receive 20 mg of atorvastatin per day or matching placebo.446 After 4 weeks of treatment, atorvastatin reduced the median LDL-C level by 42%, and placebo, by 1.3%. At least 1-mmol/L difference in LDL-C level was maintained throughout the treatment period (Fig 20) . During a median follow-up of 4 years, 469 patients (37%) reached the primary end point (a composite of cardiac death, nonfatal myocardial infarction, and fatal and nonfatal stroke): 226 assigned to atorvastatin and 243 assigned to placebo (RR, 0.92; 95% CI, 0.77 to 1.10; P = 0.37; Figure 20). Atorvastatin had no effect on the single components of the primary end point with the exception of fatal stroke, in which RR was 2.03 (95% CI, 1.05 to 3.93; P = 0.04). Secondary end points, such as all combined cardiac events (RR, 0.82; 95% CI, 0.68 to 0.99; P = 0.03), were reduced, but not all combined cerebrovascular events (RR, 1.12; 95% CI, 0.81 to 1.55; P = 0.49) or total mortality (RR, 0.93; 95% CI, 0.79 to 1.08; P = 0.33).100
Despite the high rate of cardiovascular events and the pronounced LDL-C lowering by atorvastatin, a significant reduction in the incidence of the composite primary end point was not achieved (patients with an LDL-C > 190 mg/dL were not included in 4D). In contrast to 4D, CARDS reported that people with type 2 diabetes who received atorvastatin had an RR for stroke of 0.52 (95% CI, 0.31 to 0.89) compared with placebo.98 The rate of fatal and nonfatal stroke decreased from 2.8% to 1.5% (39 versus 21 patients), whereas in the 4D, it increased from 7.0% to 9.7% (44 versus 59 patients). This unexplained finding of an increase in fatal stroke requires further study. The 4D is the first large-scale cardiovascular outcome trial that does not show overall benefit from administration of a potent dose of a statin and does not confirm the generally accepted assumption that for every 30-mg/dL change in LDL-C level, the RR of coronary heart disease is changed in proportion by about 30%. The result is in accordance with observational data in patients on hemodialysis therapy that do not link dyslipidemia with reduced survival; opposite trends have been noted.447 4D results are in contrast to an observational retrospective analysis of hemodialysis patients in the US Renal Data System (USRDS) Morbidity and Mortality Study, Wave 2,448 which indicated that the risk of cardiovascular death decreases by 36% in statin users compared with nonusers. This finding illustrates the difficulty associated with basing treatment decisions on uncontrolled observational studies.448,449 Perhaps the pathogenesis of cardiovascular events in patients with diabetes on hemodialysis therapy may be different, at least in part, from that in patients without CKD stage 5. Additional information on the inverse association between cholesterol concentration and mortality is presented in the NKF-KDOQI™ CPGs for CVD in Dialysis Patients.10
Dyslipidemia may increase albuminuria and accelerate progression of DKD. Whether treatment with statins slows progression of DKD is uncertain. (Weak)
A number of observational studies have reported that dyslipidemia is associated with decreased kidney function in the general population and in patients with CKD, with or without diabetes, as extensively reviewed in the NKF-KDOQI™ CPGs for Managing Dyslipidemia in CKD.6 An analysis of the lipid profile of the DCCT/EDIC cohort of patients with type 1 diabetes demonstrated a specific profile in DKD that is characterized by high triglyceride levels, predominantly in the very-low-density-lipoprotein (VLDL) subclasses. In men, high intermediate-density lipoprotein (IDL), high LDL particle concentration, and a shift from larger toward smaller LDL-C, apolipoprotein B, and small (noncardioprotective) HDL-C particles were associated with CKD.439 In the RENAAL Study, the RR of reaching the primary composite end point (doubling of serum creatinine level, CKD stage 5, or death) among patients in the upper quartile of the distribution for total cholesterol and LDL-C was significantly higher than for those in the lower quartile.450
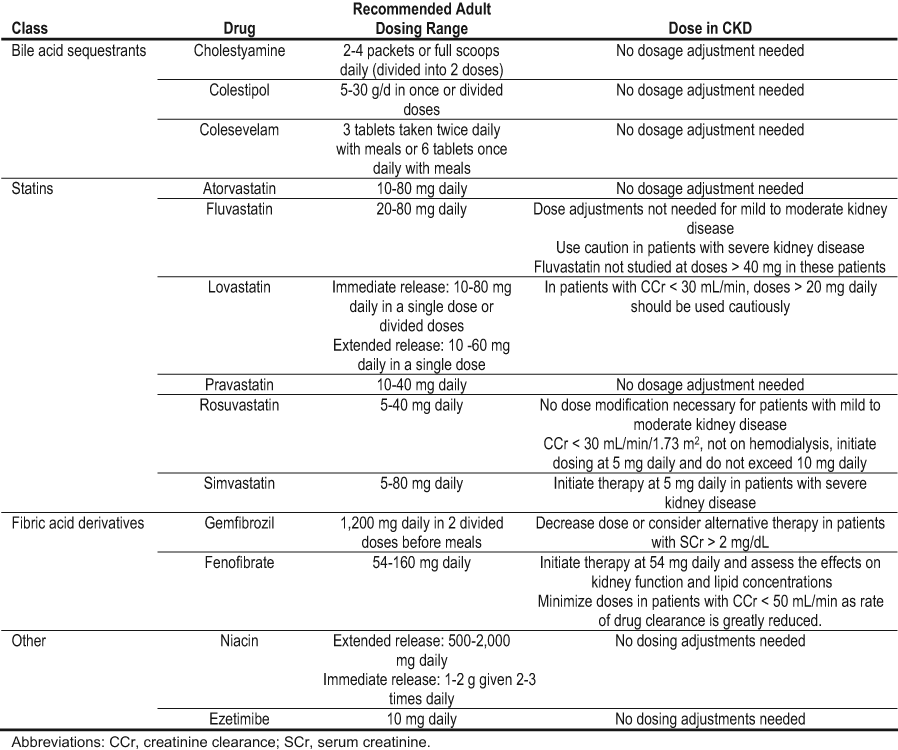
Small short-term randomized studies report mixed results of the effect of statins on progression of DKD (Table 34). In patients with type 1 diabetes and microalbuminuria, simvastatin had no beneficial effect on either albuminuria or GFR.451 However, some randomized trials in type 2 diabetes reported beneficial effects of statins on albuminuria and GFR relative to pretreatment levels,452-454 but not relative to placebo or an alternative class of treatment for dyslipidemia.455-457
Whether dyslipidemia causes reduced kidney function, results from reduced kidney function, or whether other conditions, such as proteinuria, cause both reduced kidney function and dyslipidemia cannot be determined from the available data. Large, double-blind, randomized, placebo-controlled, clinical trials that examine the effect of dyslipidemia treatment on progression of DKD have not been done. This is the only approach that can adequately test the hypothesis that treatment of dyslipidemia provides benefit for kidney outcomes. At present, primary and secondary prevention of CVD is the principal reason to evaluate and treat dyslipidemia in patients with diabetes and CKD.
For patients with type 2 diabetes who are taking statins, routine monitoring of liver function tests or muscle enzymes is not recommended except in specific circumstances. (Strong)
The current literature suggests that statins are safe. Although discontinuation and nonadherence rates are approximately 15% or more in many clinical trials, rates of discontinuation typically do not differ from those of placebo. Rates of elevated liver or muscle enzyme levels did not differ between the statin and placebo groups in the 4D or in recent large-scale studies in people with and without diabetes from the general population. Ongoing large-scale trials in diabetic and nondiabetic CKD and dialysis patients (A study to evaluate the use of Rosuvastatin in subjects on regular hemodialysis: an assessment of survival and cardiovascular events [AURORA]; and Study of Heart and Renal Protection [SHARP]) have already accumulated substantial patient treatment years and have not reported serious adverse events related to liver or muscle function. On the basis of the safety data pertaining to these drugs, routine monitoring of muscle enzymes and liver function tests probably is not warranted unless patients have symptoms,458,459 have baseline abnormalities of liver function test results or myopathy, or are taking other drugs that interact with statins to increase the risk of adverse events.
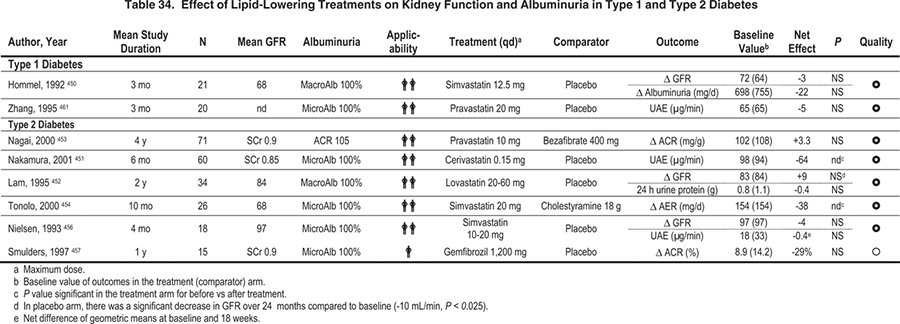
Table 35. Dosing Adjustments of Medicines to Treat Lipid Disorders in CKD
The 2004 NCEP Report on ATP III Guidelines
A recent NCEP report discussed the implications of the ATP III guidelines.175 Results from the HPS and the Pravastatin or Atorvastatin in Evaluation and Infection Therapy (PROVE IT) Study suggested that additional benefit may be obtained by reducing LDL-C levels to less than 100 mg/dL. While awaiting the results of the Treating to New Targets (TNT) Study to prove the efficacy of lowering LDL-C to very low levels, the NCEP report stated that “until these trials are completed, prudence requires that setting an LDL-C goal of <70 mg/dL for high-risk patients must be left as a therapeutic option on the basis of clinical trial evidence, whereas a goal of <100 mg/dL can be retained as a strong recommendation.” Since that NCEP report, TNT has been completed and showed that intensive lipid-lowering therapy reaching a mean target LDL-C level of 77 mg/dL with 80 mg of atorvastatin per day in patients with stable coronary heart disease provides significant clinical benefit beyond that afforded by treatment with 10 mg of atorvastatin per day.460 Factors that favor a decision to reduce LDL-C levels to less than 70 mg/dL are those that place patients in the category of very high risk.175
Table 36. NKF-KDOQI™ CPGs for Managing Dyslipidemia in CKD Stages 1 to 46

Based on these data, it is the opinion of the Work Group that people with type 2 diabetes and CKD stages 1 to 4 may receive additional benefit from intensified treatment with a statin to reduce LDL-C levels to less than 70 mg/dL. Data from the 4D suggest that initiating lipid lowering with a statin in hemodialysis patients with type 2 diabetes may come too late to translate into substantial improvement in cardiovascular outcomes, possibly because of additional or alternative pathological mechanisms.
These guideline recommendations should be validated in people with diabetes and CKD stage 4. All available studies systematically excluded people with severe impairment of kidney function. In addition, there are no prospective randomized controlled trials available in diabetes and CKD stages 1 to 3. Recommendations made for patients in the latter stages of CKD have been made based on post hoc analysis with limited numbers of patients. Results from ongoing prospective randomized trials, such as the SHARP, are eagerly awaited. The guideline recommendation for the therapeutic option of an LDL-C goal less than 70 mg/dL was based on the very high CVD risk in people with diabetes and CKD. However, the clinical trial evidence for this goal is based on studies of patients with coronary heart disease. Studies of the target population (diabetes and CKD stages 1 to 4) are needed to verify this recommendation. Data from AURORA are needed to prove whether a recommendation to withhold statin treatment in patients with type 2 diabetes on hemodialysis is justified.
Dosing adjustments of statins and fibric acid derivatives may be required in patients with diabetes and advanced CKD (Table 35). Ezitimibe may be considered as an adjunct to achieve LDL-C goals, particularly if statin doses are limited due to concern about side effects.
In the opinion of the Work Group, some caveats should be considered for implementing recommendations made in this guideline. First, the 4D results may not apply to patients who are already on drug therapy for LDL-C. Therefore, statin treatment may continue into CKD stage 5 if initiated at an earlier CKD stage. Second, the 4D results do not preclude statin treatment for hemodialysis patients with type 2 diabetes and LDL-C >190 mg/dL or specific cardiovascular indications. According to ATP-III these indications include coronary heart disease and coronary heart disease risk equivalents (eg, non-coronary atherosclerotic disease or multiple risk factors that confer a 10-year Framingham risk >20%).
Assessment of Lipids
A complete lipid profile, including total cholesterol, HDL-C, and triglycerides, should be directly measured in people with diabetes and CKD. LDL-C is calculated from these values by using the Friedewald formula, as recommended by the ATP III Guidelines.175 Because dyslipidemia associated with diabetes and CKD may occur without an elevated LDL-C level due to increased lipoprotein remnants, non–HDL-C (total cholesterol – HDL-C) also is a useful measure.175 The NKF-KDOQI™ CPGs for Managing Dyslipidemia in CKD recommend assessment of the lipid profile at least annually in people with CKD stages 1 to 4.6 Repeated measurements should be made 2 to 3 months after starting or adjusting lipid-lowering treatment or with substantial changes in albuminuria/proteinuria or GFR (Table 36).