Selection of dialyzer membranes and reuse practices are not included in the prescription of small-solute clearance, yet they can be important determinants of patient survival and QOL.
5.1 When dialyzers are reused, they should be reprocessed following the Association for the Advancement of Medical Instrumentation (AAMI) Standards and Recommended Practices for reuse of hemodialyzers.291<
5.2 Dialyzers intended for reuse should have a blood compartment volume not less than 80% of the original measured volume or a urea (or ionic) clearance not less than 90% of the original measured clearance.
5.3 The use of poorly biocompatible, unmodified cellulose dialyzer membranes for HD is discouraged.
Hemodialyzer Reprocessing and Reuse (CPR 5.1)
Thorough examination of data pertaining to the impact of reused dialyzers on patient safety was beyond the scope of the HD Adequacy Work Group. Therefore, the Work Group takes no position for or against the practice of dialyzer reuse.
Reprocessing dialyzers for reuse in the same patient was popularized 2 to 3 decades ago to allow widespread use of the more biocompatible and higher flux dialyzers that are more expensive than their less biocompatible and lower flux counterparts. Reuse of the former more expensive dialyzers remains a common practice in the United States today.41,292-297 In 2002 in the United States, 78% of HD clinics reprocessed dialyzers,41 but—largely as a result of declining prices and the recent decision of a major dialysis provider (Fresenius Medical Care, US) to discontinue reuse—fewer US dialysis patients are enrolled in reuse programs today.
Reprocessing of disposable medical devices designed for single use as a cost-saving measure has been debated, not only for dialyzers, but also for sundry and other medical devices.297 In the case of dialyzer reuse, the main concern has been the risk to life, but other issues have been raised, such as risk for infection and pyrogenic reactions, toxicity from disinfectants, reduced dialyzer performance,297 impaired removal of large molecules,294 and the validity of the dialyzer blood volume measurement as a criterion for assessing dialyzer function.292,298
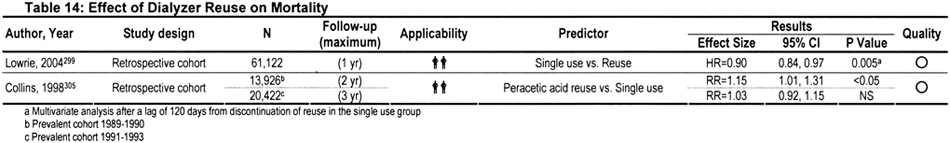
Over the years, a plethora of publications have addressed the possible cause-and-effect relationship between reuse and mortality. Conclusions reported in earlier publications were conflicting, possibly because reuse-related morbidity and mortality is a moving target (Table 14). Practice patterns, reuse procedures, dialyzer membranes, comorbidity, age difference, nature of the primary disease, disease severity, ethnic make-up, and other potentially confounding influences have evolved over time. For example, high-flux synthetic membranes have almost completely replaced low-flux cellulosic membranes. Whereas the number of times that a dialyzer is reused varies from clinic to clinic, the average number of reuses per dialyzer is higher (>15) in recent years compared with earlier years (<10).294 The sterilant used also has varied from clinic to clinic and over time. During 1983 to 2002, the percentage of centers using formaldehyde for reprocessing dialyzers decreased from 94% to 20%, whereas the percentage using a peracetic acid preparation increased from 5% to 72%. In 2002, a total of 4% of centers used heat or glutaraldehyde to disinfect dialyzers between reuses.295 Also, the number of times that a dialyzer is reused varies from clinic to clinic. Because of these various confounding factors, research data obtained from decades-old studies may have less present-day clinical relevance.
In one of the largest retrospective analyses, 1- to 2-year follow-up data were examined in a representative sample of 12,791 patients treated in 1,394 dialysis facilities from 1994 through 1995.297 After adjustment for other risks, RR for mortality did not differ for patients treated in clinics that reused dialyzers compared with patients from single-use clinics. In addition, among patients at clinics that reused dialyzers, high-flux synthetic membranes were associated with lower mortality risk, particularly when exposed to bleach.297 However, a recent study found a patient survival advantage when the patient was switched from reuse to single use.299 It was suggested that because the cost of biocompatible membranes has decreased of late, it might be time for dialysis clinics to consider abolition of the reuse practice.300 However, the cost of single-use biocompatible dialyzers is still considerable, and most investigators continue to maintain that the practice of reuse is safe,301,302 provided it is performed according to recognized reuse protocols, including the dialyzer manufacturer's instructions.292,295,296,303
In an analysis of 49,273 incident Medicare patients from 1998 to 1999, no significant differences in mortality or first hospitalization risk were found among patients treated with single-use dialyzers compared with dialyzers cleansed by using different reprocessing techniques.238 In a recent review of published reports, adjusted Medicare and Centers for Disease Control data from the early to mid-1990s showed no measurable mortality risk from reuse.239 In accordance, recent Medicare data also showed no survival advantage associated with single use in incident US patients during 2001.304 In addition, no differences in mortality were found among for-profit, not-for-profit, hospital-based, and free-standing clinics. To date, no prospective RCTs of dialyzer reuse have been carried out.
The delivered dose of dialysis may decrease as a result of dialyzer reuse.306-310 The previous Work Group was particularly concerned by the apparent dialysis center–specific effect of reuse on delivered Kt/V, suggesting that the process of dialyzer reuse and/or its monitoring may be problematic. Recently, more encouraging results generated by the HEMO Study showed that average loss of urea clearance was only 1% to 2% per 10 reuses for both low-flux and high-flux membranes reprocessed with different germicidal regimens.310 Focusing on larger molecule removal, the same study showed that reuse of high-flux dialyzers made of different membrane materials and reprocessed with different germicides brought about widely disparate clearances of β2M.310 For example, β2M clearances increased markedly by using high-flux polysulfone dialyzers reprocessed with bleach, whereas reprocessing the same dialyzer with peracetic acid appeared to have the opposite effect.310
The Work Group recommends that dialysis facilities choosing to reuse dialyzers follow the AAMI recommendations for reprocessing while remaining alert to the possibility that reuse may adversely affect adequacy of the delivered dialysis dose. AAMI recommendations were prepared by a panel of experts and offer practical reuse procedures that have been adopted by the CMS, formerly Health Care Financing Administration. These recommendations represent the best guidance available on dialyzer reuse procedures.
Monitoring Reuse (CPRs 5.1 and 5.2)
Because small-solute clearance is the major function of the dialyzer and clot formation within the blood compartment reduces clearance, sometimes irreversibly, a method for monitoring clearance with each reuse is required to avoid underdialyzing the patient. Dialyzer blood compartment volume, sometimes called “total cell volume” (TCV) or “fiber bundle volume,” is an indirect measure of the total membrane surface area available for diffusive transport. It is measured easily by displacement of air or water during the reprocessing procedure.291 As surface area is lost because of clotting, solute clearances decrease, putting the patient at risk for underdialysis. This risk would go undetected in a clinic that does not measure clearances or TCV with each reuse.306,308-311 Changes in TCV were shown to correlate well with changes in small-solute transport characteristics of hollow-fiber dialyzers, although the relationship is not linear.307,312,313
A 20% loss of TCV correlates with only a 10% loss of clearance because the (now) higher velocity in the remaining functioning fibers leads to an increase in average diffusion rate within each fiber.291,313,314 To allow accurate measurement of these changes, TCV should be measured before the first use and during each subsequent reuse processing. The first measurement is required because of possible variability among dialyzers and dialyzer lots. The Work Group did not consider using the average volume among dialyzers of a given model or lot as an acceptable substitute for this measurement before first use.
In vitro determination of TCV may not detect loss of surface area caused by clotting during dialysis.315 However, during routine dialysis in a representative group of patients who underwent adequate anticoagulation during each dialysis treatment, no differences were found between TCV values measured by using an ultrasound detection method applied during dialysis and conventional volume displacement measurement after dialysis.316
In the place of TCV as an indirect yardstick of dialyzer function, direct measurements of ionic clearance (also known as conductivity or sodium clearance) or urea clearances also can be used to evaluate dialyzer function because results of these clearance values correlate closely with one another and TCV results.74,291,317-323 A variety of dialysate delivery systems have the capacity to perform noninvasive, automated, on-line determination of a dialyzer's ionic clearance.318,319,323 The Work Group agrees with the AAMI that TCV, ionic clearance, and urea clearance can all be used to assess the function of either fresh or reused dialyzers.76,317
Because a 10% decrease in urea clearance could lead to inadequate dialysis if the dialysis prescription was marginal to begin with, the Work Group agrees with the position of the AAMI that a change in urea clearance of ±10% is acceptable as long as the patient's dialysis prescription takes into account the 10% loss in such clearance (20% loss in TCV) that may occur with dialyzer reuse.291 This criterion of ±10% clearance change also should apply to ionic clearances when they are used as yardsticks because ionic clearance was shown to correlate closely with urea clearance.291 Finally, monitoring relevant patient data is recommended to ensure that all parameters relating to dialyzer clearance are being met. Specifically, examination of Kt/V and/or URR over time is needed. The failure of these results to meet the expectations of the dialysis prescription should be investigated.291
When TCV measurements are used to evaluate dialyzer function before the first use, the rinsing associated with the reprocessing procedure may help remove undesirable dialyzer residuals (such as ethylene oxide,324 bore fluids, potting compound [eg, polyurethane] fragments, dialyzer membrane fragments, plastic components, and other noxious substances remaining after dialyzer manufacture). In this regard, it is now a not-uncommon practice for centers (regardless of whether practicing dialyzer reuse) to “preprocess” dialyzers before their first use to minimize the introduction of harmful manufacturing residuals into the bloodstream.300
Dialyzer Membranes (CPR 5.3)
Dialyzer membranes can be classified into low-flux or high-flux varieties in accordance with their ultrafiltration coefficient (Kuf) and large-molecule clearance. The HEMO Study suggested that membranes with β2M clearance less than 10 mL/min be regarded as low flux, whereas those with β2M clearance greater than 20 mL/min and Kuf of 14 mL/h/mm Hg or greater may be classified as high flux.270 Another classification recommended that dialyzers with Kuf between 4 and 8 mL/h/mm Hg be regarded as low flux, whereas those with Kuf greater than 20 mL/h/mm Hg be regarded as as high flux.325 Both cellulose and synthetic membranes can be either low flux or high flux.
A thorough examination of all available data concerning the pros and cons of the use of the myriad varieties of dialyzer membranes was beyond the scope of the Work Group. The reader is referred to standard texts and relevant publications for more information.
In the past, most cellulose membranes were primarily hydrophilic and synthetic membranes were primarily hydrophobic. However, more recent synthetic membranes can possess mixed hydrophobic-hydrophilic structures.325 Unmodified cellulose dialyzers had enormous popularity in the past, mainly because of their availability and low cost, but their use has been associated with a variety of abnormal biochemical changes in the blood.326 One of the main causes for these unfavorable changes centers on activation of the alternate complement pathway with the resultant formation of detrimental anaphylatoxins.327 Other adverse effects involve impairment of granulocyte function, including phagocytosis, adhesion, and formation of reactive oxygen species,328 and, in the presence of other factors, facilitation of cytokine production by peripheral-blood mononuclear cells. An example of the latter phenomenon is depicted as follows: unmodified cellulose membranes and certain modified cellulose membranes allow, by diffusion, more ready passage of pyrogens (eg, endotoxins and their fragments) into the blood from contaminated dialysate than such synthetic high-flux membranes as those of polyamide, polyacrylonitrile, and polysulfone—despite the larger pore size of the high-flux membranes.329,330 Pyrogens can promote the formation of deleterious cytokines by circulating peripheral-blood mononuclear cells that previously were stimulated by exposure to unmodified cellulose membranes.331,332
With regard to the possible impact of the use of unmodified cellulose membranes on patient morbidity and mortality, suffice it to say that investigations carried out to date provided conflicting results.333 A number of studies suggested that low-flux unmodified cellulose membranes are inferior to high-flux synthetic ones in terms of patient mortality.297,328,334,335 Conversely, no differences in mortality were found in certain comparative studies.280,336 Furthermore, the Cochrane Database of Systematic Reviews did not find evidence of benefit when synthetic membranes were compared with cellulose or modified cellulose membranes with regard to mortality and dialysis-related adverse effects.237 Finally, in patients dialyzed with unmodified cellulose membranes, no acute clinically detectable ill effects that could be related to complement activation were observed.337,338 Investigations that control for the confounding influences of age, sex, race, duration of renal failure, duration and type of prior dialysis treatments, primary disease, RKF, nutrition status, degree of fluid overload, calcium × phosphorus product, hyperparathyroidism, hyperlipidemia, acidosis, anemia, comorbidities (such as diabetes, hypertension, heart failure, and other cardiovascular ailments), dialyzer single use or reuse (if reuse, method of sterilization), membrane flux, dialysis adequacy, and so on are difficult to perform. Such confounders might help explain the conflicting results encountered to date. In summary, to date, no unequivocal evidence has come forward supporting the notion that biocompatible synthetic membranes are definitely superior to their less biocompatible cellulose-derived counterparts.
Not all cellulose membranes behave in the same manner when interacting with the body. For example, unmodified cellulose membranes activate complement to a greater extent than modified cellulose membranes, such as those of various cellulose acetates, whereas some of the modified cellulose membranes tend to activate complement to a greater extent than synthetic membranes.339,340 Because of differences in the biological behavior of the various categories of cellulose membranes, data derived from the use of functionally diverse dialyzers should be evaluated separately.
Many synthetic membranes have the capacity to adsorb endotoxins and β2M to various extents. Adsorption of endotoxins is related to the provision of binding sites for bacterial products by the hydrophobic domains of the synthetic membranes.329 Adsorption of β2M by membranes made of polysulfone, polyacrylonitrile, polyamide, polymethylmethacrylate, and polycarbonate279 is believed to be a function of the electrical charges distributed both at the surface and in the substance of the membrane.341,342 It should be noted that high-flux membranes (whether cellulose or synthetic), because of their greater porosity, remove such large molecules as β2M (molecular weight, 11,815 d) to a greater extent than low-flux cellulose or low-flux synthetic membranes, often decreasing serum levels.277,280,343-346 Accumulation of β2M in high concentrations promotes its polymerization to cause β2M amyloidosis.
Use of high-flux synthetic polyacrylonitrile membranes has brought about a lesser incidence of the amyloid-associated carpal tunnel syndrome and cystic bone lesions than the use of low-flux cellulose membranes.282 Furthermore, high-flux dialysis using polysulfone membranes was reported to postpone clinical manifestations of dialysis-related amyloidosis.347 In 1 study, prolonged use of high-flux synthetic membranes led to improvement in carpal tunnel syndrome and patient mortality.348 In the HEMO Study, although high-flux membranes did not cause a statistically significant improvement in mortality, predialysis serum β2M levels were found to be a good predictor of mortality.349
Because unmodified cellulose membranes have no known advantages over synthetic membranes other than lower cost, and unmodified cellulose membranes can markedly activate complement and bring about other potentially adverse effects in the blood, it would seem prudent to dialyze patients with the more biocompatible and less complement-activating membranes.279 This suggestion is strengthened because long-term effects of intense complement activation and other untoward interactions with blood are largely unknown. However, it equally could be argued that because of their lower costs, unmodified cellulose dialyzers would allow the implementation of otherwise cost-prohibitive, but life-saving, dialysis therapy in some developing countries.350 Because synthetic membranes are more biocompatible, cause less complement activation, and can adsorb endotoxins and β2M, their use is favored.