Catheters and ports are essential tools for providing urgent and, in some cases, long-term vascular access. Prevention and early treatment of complications should greatly reduce associated morbidity and mortality.
7.1 Catheters and ports should be evaluated when they become dysfunctional. Dysfunction is defined as failure to attain and maintain an extracorporeal blood flow of 300 mL/min or greater at a prepump arterial pressure more negative than –250 mm Hg. (B)
7.2 The exception is pediatric or smaller adult catheters that are not designed to have flows in excess of 300 mL/min. (B)
7.3 Methods that should be used to treat a dysfunctional or nonfunctional catheter or port include:
- 7.3.1 Repositioning of a malpositioned catheter. (B)
- 7.3.2 Thrombolytics, using either an intraluminal lytic, intradialytic lock protocol, or an intracatheter thrombolytic infusion or interdialytic lock. (B)
- 7.3.3 Catheter exchange with sheath disruption, when appropriate. (B)
7.4 Treatment of an infected HD catheter or port should be based on the type and extent of infection.
- 7.4.1 All catheter-related infections, except for catheter exit-site infections, should be addressed by initiating parenteral treatment with an antibiotic(s) appropriate for the organism(s) suspected. (A)
- 7.4.2 Definitive antibiotic therapy should be based on the organism(s) isolated. (A)
- 7.4.3 Catheters should be exchanged as soon as possible and within 72 hours of initiating antibiotic therapy in most instances, and such exchange does not require a negative blood culture result before the exchange. (B) Follow-up cultures are needed 1 week after cessation of antibiotic therapy (standard practice).
- 7.4.4 Port pocket infections should be treated with systemic antibiotics and irrigation, in conjunction with the manufacturers' recommendations. (B)
Evaluation of Dysfunction (CPG 7.1)
Catheter dysfunction can be attributed to many causes, and progression of dysfunction to nonfunction varies accordingly.182 The most common complications are thrombosis and infection.486,487 Even with care, fewer than half the catheters placed as “long-term access” are in use a year after their placement,488 and about a third are removed because they fail to deliver adequate blood flow. The definition of adequate blood flow varies inversely with the “efficiency” of HD. High-efficiency dialysis as practiced in the United States requires dialyzer-delivered BFRs greater than 300 mL/min to achieve the target single-pool Kt/V of 1.2 (see the KDOQI HD Adequacy Guidelines). Conversely, in Europe, BFRs less than 300 mL/min frequently are used because dialysis treatment durations are longer.203 Adequacy of dialysis is influenced additionally by the site of placement and degree of recirculation.489,490 Recirculation in femoral catheters is significantly greater than that in internal jugular catheters (13.1% versus 0.4%; P < 0.001).193 In addition, femoral catheters shorter than 20 cm have significantly greater recirculation (26.3%) than those longer than 20 cm (8.3%; P = 0.007). This length dependency may result from the ultimate tip position of longer catheters in the IVC as opposed to the common iliac vein with shorter catheters. The greater blood flow available to the catheter at the IVC site reduces recirculation. When dialysis dose delivery is a priority, placing the short-term catheter in the internal jugular vein is an advantage. Recirculation may increase when the “lines are reversed” (inversion of inlet and outlet lumens), even in “well functioning” nonsplit catheters (from 2% to 3% to >10%).491 Although reversal of tubings may increase urea clearance by increasing blood flow temporarily,184 it usually is at a BFR less than 300 mL/min and should never be used except temporarily until the problem is definitively corrected.
A dysfunctional catheter usually is easier to salvage than a nonfunctional catheter, thereby preventing complications of a new placement.249 Early treatment also reduces the likelihood and minimizes the extent of inadequacy of dialysis caused by catheter dysfunction. Delivery of adequate dialysis dose is dependent upon blood flow and treatment duration. For any given dialyzer, low BFRs during HD extend treatment times and all too often still result in underdialysis (caused by unrecognized recirculation). A BFR less than 300 mL/min was noted in 15% of treatments with catheters.249 Catheter dysfunction leads to 17% to 33% of untimely catheter removals,487,488 and thrombosis of the catheter occurs in access loss in 30% to 40% of patients.
It is to be noted that the criterion for determining access dysfunction, ie, blood flow greater than 300 mL/min, is qualified by the prepump arterial pressure182 factored for the length and lumen diameter of the catheter.183,490 Prepump arterial pressure monitoring is essential to ensure valid blood flows, and adequacy is determined largely by the amount of blood pumped to and through the dialyzer.189,191,200 Consequences of catheter dysfunction are many, including increases in morbidity and mortality,20,248,258 increase in economic expenditures,250 and a “real” concern to patients, 60% of whom report fear of thrombosis second only to pain in decreasing their QOL.252
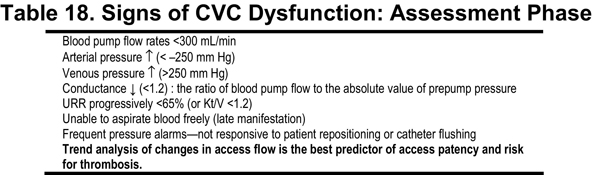
In CVCs, the most likely cause for low BFRs is thrombotic occlusion. In the likely event that low BFR or occlusion will occur at some time during the useful life of a catheter, prospective monitoring is essential to detect dysfunction. Regular assessment of dialysis performance is strongly recommended to ensure dialysis adequacy.189 Catheter performance parameters to consider are shown in Table 18 and include maximal consistently achievable BFR, resistance to blood flow indicated by arterial and venous pressures during HD, and blood recirculation rate.492,493 Of these, the one favored by the Work Group is the ratio of dialyzer BFR achieved, factored by the prepump arterial limb pressure in absolute units.
Early detection of access dysfunction is most likely if all members of the VAT are involved.198 The use of CQI in catheter access necessitates collaboration among team members, with specific tasks assigned to certain individuals, who then provide input and/or feedback.
The minimally accepted dialyzer BFR of 300 mL/min is easily achieved by using newer catheters that are capable of achieving rates of 400 mL/min or greater when properly placed.494 Therefore, 300 mL/min is a conservative value in current adult practice and waiting until blood flow decreases to 300 mL/min may be too late to avoid loss of the catheter and unnecessary loss of the access site.
Prevention of catheter and access thrombosis by using antiplatelet agents and anticoagulation has not been successful (see Table 19) .
Use of an antiplatelet agent is not recommended because it was not effective in grafts and was associated with more bleeding.497 A similar conclusion was reached in a prospective nonrandomized comparison of warfarin to aspirin.498 Use of a low fixed dose (1 mg) of warfarin also was found to be ineffective.495 Further studies in this area with a higher target international normalized ratio (INR) are warranted.
The first step in assessing dysfunction is shown in Fig 9, which begins with a determination of the age of the catheter.
Fig 9. Assessing dysfunction of catheters. Symbols: IR, imaging for correct position. Abbreviation: tPA, tissue plasminogen activator; IR, intervention. (Courtesy of Drs Asif and Anatole Besarab).
In catheters recently placed, inadequate blood flow usually is the result of mechanical obstruction, improper tip location affected by patient position, or a problem of catheter integrity, as shown in Table 20.492
The need to use a Trendelenberg position to achieve adequate blood flow from a catheter placed in great veins leading to the right atrium always implies that the catheter is improperly placed. If the problem is not obvious and not easily correctable, the patient should be referred to an interventional center for study to diagnose the cause. Although mechanical problems can develop acutely in catheters previously giving good performance, access dysfunction occurring after 2 weeks more likely is the result of progressive occlusion of the catheter tip by fibrin or thrombus. The location of obstruction may reside in the following areas:
Methods That Should be Used to Treat a Dysfunctional or Nonfunctional Catheter or Port (CPG 7.3)
A catheter that has migrated out of the mid-right atrium should be repositioned. Catheters of inadequate length should be exchanged over a guide wire to the appropriate position or replaced.499
All catheters are “locked” with some anticoagulant. The purpose of the lock is to prevent thrombosis. Loss of anticoagulant by diffusive transport would be expected. However, it has been known for several years that some fraction of the anticoagulant will leak into the systemic circulation500,501 by nondiffusive processes and increase the partial thromboplastin time, thus possibly contributing to minor or even major bleeding. In vitro simulations suggest an early leak within 30 seconds, followed by a slower loss of locking solution during the next 30 minutes.502 The specific gravity of the locking solution likely also influences the rate of leak.503 Locking solution lost is replaced by blood. It therefore is not surprising that thrombosis is so common in catheters because blood is likely to be in the lumen of the catheter for prolonged times interdialytically. The loss of anticoagulant permits the entry of clotting factors into the catheter lumen. The presence of these processes is manifested by a change in flow long before there is occlusion.
After assessment precludes mechanical dysfunction (see Fig 9), such as a kink or dislodgement, thrombotic occlusion—partial (poor flow on aspiration) or total (unable to aspirate or push)—is the most common cause of catheter dysfunction and/or occlusion.151,504-506 Pharmacological intervention for catheter-occlusive dysfunction involves treatment with thrombolytics that convert plasminogen to plasmin. Thrombolytics are noninvasive, confer no additional trauma to the patient, have a high level of safety and efficacy, and are cost-effective.507 A thrombolytic can be administered in the dialysis setting. Because of such advantages and the less practical alternative treatment options, thrombolytic therapy directed at salvaging the catheter should be considered before access replacement because it is the least invasive and least costly of all catheter salvage techniques.
A variety of thrombolytics have been used (Table 21), although at the present time, only tissue plasminogen activator (tPA) is approved by the Food and Drug Administration (FDA). Urokinase (UK) is still available (but is no longer manufactured), as is reteplase, but neither of these lytics currently is available in convenient dosages and must be aliquoted and frozen for use. Teneplase, another lytic, has not been used for access thrombosis. Formulations of some lytics have been tried in studies and are used “off label” at various institutions.
Thrombolytics have proved highly effective in opening partially and fully occluded lumens.496,508-525 (See also: Abbott Laboratories, prescribing information for abbokinase [urokinase] Chicago, IL, 2003; Boehringer Mannheim GmbH, prescribing information for Reteplase® [reteplase], 2000; Genentech, prescribing information for Cathflo® Activase® [alteplase], 2001.525A-C) The most common use of lytics occurs late in the “dysfunction process,” when prescribed blood flows are not attained and there is difficulty even in initiating a dialysis treatment. Currently, the package insert only describes the use of the agent for catheters in a timed dwell, based on clinical trials in nondialysis catheters.526,527 The recent Cathflo Activase Pediatric Study has led the FDA to approve tPA as a thrombolytic in all age groups for the same indications as in the package insert.
In situations in which the obstructive process has progressed to a more severe state, dialysis is urgent, and the catheter is extremely dysfunctional (ie, unable to provide a BFR of 200 mL/min), tPA reconstituted appropriately and instilled at a lumen fill volume permits resumption of dialysis in 50% to 90% of instances (see Table 22), although a second dwell may be required. Per the package insert, this lytic should be allowed to dwell for 1 hour or longer. Table 23 summarizes the major studies with tPA in totally occluded catheters.
In general, efficacy increases with longer dwell times with tPA as the lytic. Fewer studies are available with the other agents, but results are similar.514,517 A recent study showed that a lower dose of 1 mg/lumen of tPA also is effective, restoring catheter patency in 72% with 1 dose, increasing to 83% with a second dose,513 values only slightly lower than with the “standard” dose of 2 mg/lumen.
The Work Group believes that the use of lytics late in the thrombosis process without adequate prior diagnostic evaluation is in itself “dysfunctional” and recommends that the procedures described in Fig 9 be used to evaluate a catheter access on a recurrent basis. Tracking the relationship of prepump pressure, VDP, and flow (see Fig 9) can alert the clinician to the development of catheter dysfunction before late manifestations set in. The emphasis for managing catheter dysfunction should shift to intervention at an earlier stage of dysfunction.
Although endoluminal brushes have been used to clear thrombi from dialysis catheters,528 there are no convincing data about efficacy and they are expensive. The Work Group does not currently advocate their routine use. Such brushes were originally developed to obtain biofilm specimens from catheters.
The management of fibrin sheaths is discussed further in CPR 7. Currently, the Work Group recommends change of catheter with disruption of the sheath by using a balloon. Fibrin sheath stripping rarely is used because of cost and increased patient morbidity.
Treatment of an Infected HD Catheter or Port (CPG 7.4)
Definitions
Exit-site infection. Inflammation confined to the area surrounding the catheter exit site, not extending superiorly beyond the cuff if the catheter is tunneled, with exudate culture confirmed to be positive.
Tunnel infection. The catheter tunnel superior to the cuff is inflamed, painful, and may have drainage through the exit site that is culture positive.
Catheter-related bacteremia. Blood cultures are positive for the presence of bacteria with or without the accompanying symptom of fever.
The Work Group recommends the following CDC definitions for catheter-related infections.
Definite bloodstream infection: the same organism from a semiquantitative culture of the catheter tip (>15 colony-forming units per catheter segment) and from a peripheral or catheter blood sample in a symptomatic patient with no other apparent source of infection.
Probable bloodstream infection: defervescence of symptoms after antibiotic therapy with or without removal of catheter, in the setting in which blood cultures confirm infection, but catheter tip does not (or catheter tip does, but blood cultures do not) in a symptomatic patient with no other apparent source of infection.
Possible bloodstream infection: defervescence of symptoms after antibiotic treatment or after removal of catheter in the absence of laboratory confirmation of bloodstream infection in a symptomatic patient with no other apparent source of infection.
Although thrombotic occlusions leading to flow delivery problems are more common than infection, catheter-related infection has emerged as the primary barrier to long-term catheter use. The greater infection rate in catheters compared with grafts and fistulae is its major limitation. Infection is the leading cause of catheter removal and morbidity in dialysis patients.148,156,201,532,533 The most recent USRDS data indicate that the rate of septicemia in HD patients continues to increase, and hospital admissions for vascular access infection doubled in the last decade.235 The use of long-term HD catheters instead of short-term catheters has not yet translated into a significant reduction in the incidence of CRB and resultant infective endocarditis in our population.234,534-539
Accurate and early diagnosis is essential. A meta-analysis of 8 different methods comparing those that do and do not require catheter removal showed that paired quantitative blood cultures from the peripheral blood and the catheter are the most accurate,540 but are not routinely performed. However, routine culture methods have negative predictive power (>99%), whereas the positive predictive value increases with the pretest probability for infection. Dialysis programs should monitor vascular access and especially catheter-related infections with attention to incidence, bacteriology, and outcomes.
Significant risk factors (P < 0.05) for bacteremic episodes include the presence of diabetes, peripheral atherosclerosis, a previous history of bacteremia, nasal carriage of Staphylococcus aureus, longer catheter use duration, more frequent UK catheter infusion, and local infection.532,537 One report identified elderly women as being more at risk.541
Infection monitoring should be in place to identify outbreaks that can result from manufacturing defects.542 A doubling of the rate is cause for concern.542 One study provides a means to standardize the reporting of vascular access infection rates.543 Analyzing nearly 40,000 dialysis sessions, infection rates were greatest among short-term catheters (recommended for in-hospital use only) and least among permanent native AVFs or synthetic grafts. Another analysis in Canada of 184 bloodstream infections in 133,158 dialysis procedures confirmed these findings.232 AVFs were associated with the lowest risk for bloodstream infection (0.2/1,000 dialysis procedures; RR increased 2.5-fold with AVGs, 15.5-fold with TCC access, and 22.5-fold with uncuffed CVC access; all P < 0.001). Significant variation in infection rates was observed among centers, even when controlling for types of access used, suggesting that access-specific infection rates within and among centers could be used to develop quality improvement. Experience with femoral TCCs has been mixed. Some reports indicated no increase in infection rate,544,545 but that has not been the experience of members of the Work Group. Even if there is no decrease in infection-free survival,545 use of femoral catheters is associated with ipsilateral vein thrombosis in about 26% of patients that necessates use of anticoagulants with uncertain effects on the upstream iliac vein (see CPG 2).
All indwelling vascular catheters are colonized by microorganisms within 24 hours after insertion.546 The formation of “biofilm” on the external and internal surface of vascular catheters is thought to have an important role in the colonization process. The biofilm is produced by a combination of host factors (eg, fibrinogen, fibrin, fibronectin, and extracellular polysaccharides) and microbial products (eg, glycocalyx or “slime”) and has a critical role in bacterial antimicrobial resistance and recalcitrant infections.547 Prevention of infection is the key first step, and the reader should consult the recommendations of the CDC.222 Although documented by a variety of methods, the relationship of thrombin sheath to infection has not been evaluated clinically. Proteins in the fibrin sheath provide adhesions for organism binding, particularly by S aureus. Whether more aggressive prevention of fibrin sheaths could reduce the infection rate is unknown. Sporadic reports suggested that concomitant use of a lytic with antibiotics could salvage more catheters.
In general, uncuffed catheters have a greater rate of infection, 3.8 to 6.6 episodes/1,000 days, compared with TCCs, with 1.6 to 5.5 episodes/1,000 days.534,542,544,548 This wide range obviously reflects differences in practice.544 Rates as low as 1/1,000 days at risk have been achieved with detailed catheter protocols.247 Programs with greater rates of infection in long-term catheters should institute CQI analysis techniques. Catheter infection usually requires replacement of the catheter in half the episodes despite antibiotic therapy.532 Systemic antibiotics used to treat bacteremia do not penetrate into the biofilm and therefore do not eradicate it,549 leading to potential treatment failures and eventual sacrifice of the catheter. Among uncuffed short-term catheters, femoral catheters have the highest infection rate, averaging 7.6 episodes/1,000 days, with more than 10% being infected by 1 week.199
Catheter exit-site infections alone usually can be salvaged with topical and oral antibiotics without the need for catheter replacement.148,149,151,550 CRB is the major reason for catheter loss156 and has been associated with substantial morbidity, including metastatic infection.159 It is a life-threatening condition requiring initial hospitalization and parenteral antibiotic therapy if the patient is clinically septic. The observation in a large trial of patients with CRB that systemic antibiotics alone were able to salvage less than 25% of catheters533 led to the commonly used “salvage of site rather than salvage of catheter” approach.551,552 Attempts to salvage the catheter in situ were associated with recurrence of infections soon after the antibiotics were discontinued.533 Conversely, studies using catheter guide wire exchange in stable patients without tunnel involvement under the cover of antibiotics alone salvaged 80% to 88% of sites without apparent ill effects.158,551,552 There is no advantage in delaying replacement of the catheter by several days.553 A decision-tree hypothetical analysis showed that TCC exchange over a guide wire reduced net charges by approximately $5,200 and $750 (US dollars in year 2000) compared with TCC salvage and immediate TCC removal, respectively.554 Expected 3-month patient survival for TCC guide wire exchange and immediate TCC removal were similar (93%), whereas survival for TCC salvage was worse.554 A negative culture result is not required before catheter exchange.551
An alternative to this management of dialysis CRB (systemic antibiotics with catheter exchange, as well as removal of the infected catheter) is catheter salvage by combining systemic antibiotics in conjunction with antibiotic locks.555-559 The former is burdensome at times and expensive and creates short-term problems for dialysis access if the infectious disease consultant demands that a 24- to 48-hour catheter-free period is needed before the catheter can be placed. As stated previously, bacterial biofilms form routinely in the catheter lumen and act as the nidus for bacteremic episodes. Instillation of a concentrated antibiotic-anticoagulant solution into the catheter lumen (antibiotic lock) at concentrations orders of magnitude higher than those achievable in the blood may permit successful eradication of the infection while salvaging the patient's catheter. A number of studies now confirm the validity of this approach,556-559 with salvage of the catheter and without recurrence of infection in about 65% to 70% of cases, comparing favorably with the catheter-exchange approach. With the latter method, catheter replacement is necessary in patients with persistent fever or positive surveillance blood culture results. A direct head-to-head RCT of the 2 methods is needed (see Research Recommendations).
Bacteremia with tunnel-tract involvement should prompt catheter removal. Unstable patients require removal of the catheter for rapid response to therapy. The Work Group believes that a minimum of 3 weeks of systemic antibiotic therapy is needed to treat CRB and that new permanent access should not be placed until culture results have been negative for at least 48 hours after cessation of antibiotic therapy.
Prevention of CRB can be difficult despite the use of rigorous infection-control techniques. As shown in Table 24 , silver impregnation of the catheter was ineffective,560 whereas a gentamycin/citrate solution561 and a taurolidine solution used as interdialytic antibiotic locks were effective.562,563 Minocycline/rifampin coating has not been tested in dialysis catheters.
The subject of antibiotic locking has been discussed extensively.564 Other pharmacological measures that may be useful for prophylaxis against CRB include application of an antimicrobial ointment (mupirocin or polysporin) to the catheter exit site.565,566 Subcutaneous port catheter devices do not reduce the frequency of CRB unless an antimicrobial solution is used with the device.567 A preliminary study showed that a topically applied “Medihoney” was as effective as mupirocin in reducing catheter infection.568 The former has a lower likelihood for selecting out resistant organisms. It unfortunately is forgotten that good practice and attention to “hub care” can significantly reduce CRB by 4-fold.247
However, with all preventive strategies other than good catheter care (see CPG 3), there are few long-term data on the development of antimicrobial resistance, and future studies are required. Until such data are available, it is unlikely that the use of such locks and ointments will receive official approval from the FDA.