
 |
NKF
K/DOQI GUIDELINES
|
II. CLINICAL PRACTICE GUIDELINES AND CLINICAL PRACTICE RECOMMENDATIONS FOR ANEMIA IN CHRONIC KIDNEY DISEASE IN ADULTS
CPG AND CPR 2.1. HB RANGE
Treatment thresholds in anemia management describe the intended goal of current treatment for the individual patient. The Hb treatment range represents the intended goal of ESA and iron therapy.
2.1.1 Lower limit of Hb: In patients with CKD, Hb should be 11.0 g/dL or greater. (MODERATELY STRONG RECOMMENDATION)
2.1.2 Upper limit of Hb:
In the opinion of the Work Group, there is insufficient evidence to recommend routinely maintaining Hb levels at 13.0 g/dL or greater in ESA-treated patients.
BACKGROUND
Previous (2001) KDOQI Guidelines for the Treatment of the Anemia of CKD2
incorporated the following lines of evidence to support a recommended
Hb target level of 11.0 to 12.0 g/dL in patients with CKD:
RATIONALE
Hb Level Should Be 11.0 g/dL or Greater
Evidence to support a recommended target Hb level demands the highest
order of methodologic rigor. To determine the recommended target Hb
level, we confined consideration to results gained from RCTs that
enrolled patients who are representative of the key CKD populations
(anemia in patients with ND-CKD, PD-CKD, and HD-CD), assigned patients
to at least 2 distinct target Hb level groups (treatment versus
placebo/control or higher versus lower Hb level), assessed outcomes
that are important to patients, and reported results of between-group
comparisons. We based our conclusions on evidence that, when taken
together, reflected high or at least moderate overall quality. Such
evidence has few limitations, shows consistency among trials, bears
direct relevance to the anemic CKD population, and lacks sparseness or
substantial bias. We gave weight to between-group differences (effect
sizes) that are not only statistically significant, but also clinically
meaningful, particularly when considering QOL. When weighing evidence
of improved outcomes against the potential for increased risk, we
sought to determine the Hb level(s) at which a patient can expect an
overall net benefit. Finally, we required that the recommended target
Hb statement be clear and unambiguous and the target Hb level be
achievable in practice.
Evidence supporting the statement that Hb level should be 11.0 g/dL or greater includes results from 22 RCTs and is presented both in detail for each trial (Table 12 through 19) and in summary for each outcome (Table 20 and Table 21).
The evidence is confined to results of between-group comparisons
generated by trials randomizing patients to distinct intent-to-treat Hb
targets, using “ESA versus placebo” or “ESA lower Hb target versus ESA
higher Hb target” designs (Fig 15). We excluded
evidence from observational studies. Cohort-based observational trials
and cross-sectional analyses of large medical databases, such as
within-group analyses in RCTs, consistently show that higher achieved
Hb values (including ≥12 g/dL) are associated with improved patient
outcomes, including lower mortality, less frequent hospitalization, and
less severe LVH. Conversely, distinct-target RCTs consistently show
that patients assigned to a Hb value of 13 g /dL or greater show no
discernable improvement in survival, hospitalization, or LVH compared
with patients assigned to Hb targets less than 13 g/dL and may be prone
to excess adverse cardiovascular events. The failure of observational
associations to be confirmed by interventional trials renders use of
observational evidence unsuitable to support the development of an
intervention guideline statement.
The statement that Hb level should be 11.0 g/dL or greater incorporates evidence from Hb targets ranging from 6 to 16 g/dL (Table 12 through Table 21; Fig 15). RCTs conducted before 1998 are characterized by small study size, higher target Hb levels within the range of 10 to 13 g/dL, and a lower target Hb level that reflects assignment to placebo or no-treatment control. Trials conducted thereafter are characterized by larger study size, higher target Hb levels within the range of 12 to 16 g/dL, and lower target Hb levels between 9 and 12 g/dL. The lesser magnitude of between-group Hb level differences seen in more recent trials is associated with Hb baseline values that are greater than those of early trials and lower target Hb level ranges that are substantially greater than Hb levels previously seen in placebo or untreated controls. In addition, mean Hb levels achieved in more recent trials frequently are at or less than the lower limit of the higher target Hb level range.
Patients in the 19 RCTs reviewed to support the statement that Hb level should be 11.0 g/d or greater clearly are representative of the anemic patients with CKD that the statement intends to address. Ten RCTs enrolled patients with HD-CKD, 1 enrolled patients with PD-CKD, 2 enrolled patients with both HD and PD-CKD (Tables 12 to 14), and 9 enrolled patients with ND-CKD (Tables 15 and 16). However, the preponderance of evidence (and thus the greatest strength of evidence) lies in the HD-CKD patient group. Although sample size and thus power, effect size, and strength of evidence generally are lower in patients with ND-CKD than patients with HD-CKD or PD-CKD, the direction of effect for key outcomes appears to be the same. Accordingly, the guideline statement is designed to address all anemic patients with CKD regardless of the presence, absence, or mode of dialysis therapy.
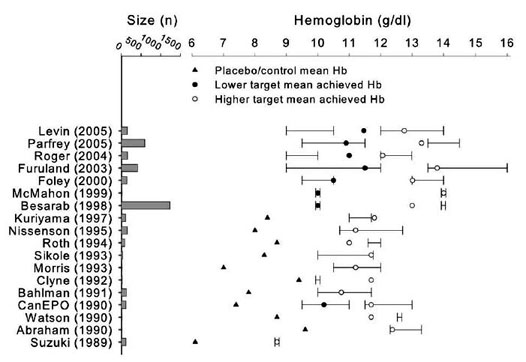
Fig 15. Target and achieved Hb levels in 18 RCTs comparing
higher against lower (or placebo/control) Hb targets for ESA therapy.
Whiskers denote the assigned (intent-to-treat) Hb target, data points
denote achieved Hb level (see legend). Not shown is Lim 1989, Berns
1999, Abraham 1991 (see Tables 12 and 14). For literature citations, see Table 12 through Table 21.
Endpoints measured to support the statement that Target Hb levels should be maintained at greater than 11 g/dL
reflect key clinical outcomes important to patients. Measured outcomes
include all-cause mortality, nonfatal cardiovascular events, LVH,
hospitalization, QOL, transfusion requirement, access thrombosis, other
thromboembolic events, seizure, blood pressure change, dialysis
adequacy in patients with HD-CKD, and kidney disease progression in
patients with ND-CKD (Table 12 and 16). Accordingly, the Work Group rated the importance of these outcomes as high or moderately high (Table 20 and Table 21).
Most distinct-target RCTs clearly have inadequate power to compare AE
rates between patients in lower and higher Hb target groups for the key
safety outcomes, mortality, MI, and cerebrovascular events (Table 12 and 16).
There are 2 exceptions. In patients with HD-CKD with CVD, patients
assigned to a Hb target level of 14 g/dL showed increased risk for
death or MI compared with patients assigned to an Hb level of 10 g/dL,108
a finding that did not reach significance (relative risk [RR], 1.3; 95%
CI, 0.9 to 1.9). However, safety concerns prompted early study
termination. In a second trial of patients with HD-CKD without
symptomatic heart disease or left ventricular dilation, patients
assigned to a Hb target of 13.5 to 14.5 g/dL showed a greater rate of
cerebrovascular events than those assigned to an Hb target range of 9.5
to 11.5 g/dL (P = 0.045).109
The risk for vascular access thrombosis in patients with HD-CKD likely
increases as the target Hb level increases, over a wide range of
potential Hb level targets. Patients assigned to Hb targets of either
9.5 to 11 or 11.5 to 13 g/dL show increased access thrombosis compared
with placebo-treated controls,49 as do patients assigned to a Hb target of 14 g/dL compared with those assigned to a target of 10 g/dL.108 Native arteriovenous fistulae fare no better than arteriovenous grafts.49,108
Substantial blood pressure increases requiring intervention are seen in
ESA-treated patients regardless of Hb target compared with those
assigned to placebo or no-ESA control groups. Increased
antihypertensive use to achieve equivalent blood pressure also has been
seen in patients assigned to higher Hb targets compared with lower Hb
targets.109, 110 However,
use of placebo and no-treatment control groups is confined to early
trials in which patients entered with low baseline Hb levels and, with
treatment, showed large relative differences between achieved Hb target
(upper or lower) and baseline Hb levels (Fig 16).
Blood pressure effects between upper and lower Hb targets appear less
prominent in recent trials compared with earlier trials; however, it is
unclear whether this difference is caused by relatively high baseline
Hb levels, small differences between baseline and either upper and
lower target achieved Hb levels, small differences between upper and
lower achieved target levels, or differences in patient selection
between early and more recent trials.
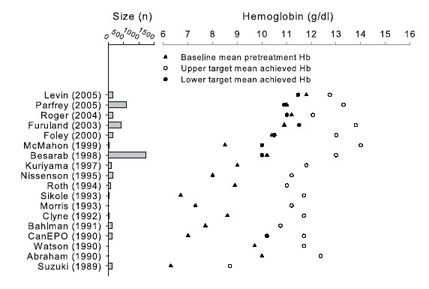
Fig 16. Baseline mean pretreatment Hb levels compared with
achieved mean Hb levels in the upper and lower target. Results for
achieved lower target Hb levels are not shown for placebo/control
groups. Data for Lim 1989 (4 target groups, n = 14 total), Berns 1999
(n = 24), and Kleinman 1989 (n = 14) not shown. In recent trials,
baseline Hb level approaches or exceeds the achieved target Hb level of
early trials, and differences between baseline and target upper
achieved Hb levels are relatively small. For literature citations, see Table 20 and Table 21.
Hospitalizations and seizures appear not to be affected by target Hb level in patients with either HD-CKD, PD-CKD, or ND-CKD (Table 20 and Table 21).
Dialysis adequacy is lower and transfusions less frequent among
patients with HD-CKD in higher-target compared to lower-target
treatment groups (Table 20).
In
patients with ND-CKD, the effect of Hb target on progression of kidney
disease is unclear. Eight RCTs enrolling more than 500 total patients
yielded inconsistent results. Individual trials showed either
prolongation of kidney survival, acceleration of progression to kidney
failure, or no effect, but many of the trials were underpowered to
detect potentially relevant effects in either direction. Because of
this limitation, the unavoidable use of compound interventions (early
versus late plus lower versus higher Hb targets), and the lack of
consistency in results, the Work Group concluded that considerations of
kidney disease progression currently should not influence the
determination of Hb targets in patients with ND-CKD.
QOL outcomes—particularly those reflecting vitality, fatigue, and
physical function—show a consistent positive relationship to level of
Hb target that appears to be continuous within the examined Hb level
range of 6 to 16 g/dL. Patients report greater vitality, less fatigue,
less depression, and improved physical symptoms at higher compared with
lower Hb targets. These results appear to be instrument dependent
because differences are more readily discernable with testing
instruments specific for CKD. Other dimensions of QOL show less
consistent results regardless of the instrument used. Although effect
size of QOL outcomes and the quality of evidence are lower in ND-CKD
compared with HD-CKD and PD-CKD trials, the direction of effect is
similar in all populations tested. The Work Group rated QOL as highly
important to patients (Table 20 and Table 21).
In developing the statement Hb level should be 11.0 g/dL or greater,
the Work Group concluded that—when comparing higher with lower Hb
targets—QOL is a sufficient and, apparently, the sole determinant of
treatment benefit. However, both the design of the RCTs supporting that
conclusion and the continuous nature of the relationship between QOL
and Hb level pose challenges to translating evidence into a guideline
statement. Hb targets in RCTs are designed to ensure separation of
treatment results in higher Hb target groups compared with lower Hb
target groups to maximize the possibility that between-group treatment
effects will be detected. These targets, whether represented as
discrete Hb values (eg, 10.0 versus 14.0 g/dL108) or a range of Hb values (eg, 9.0 to 10.0 versus 12.0 to 13.0 g/dL38),
(1) were not intended to serve as Hb targets for anemia management in
clinical practice, (2) are ill suited to the purpose of measuring
clinical performance, and (3) if adopted as a CPG, would discourage
flexibility in meeting the needs of individual patients. Moreover,
because the relationship between QOL and Hb level that emerges from RCT
results is continuous, there is no specific threshold Hb value to
distinguish between the presence and absence of benefit.
The Work Group considered, but rejected, identifying a discrete Hb
value (eg, 11.0 g/dL) as a Hb target. A discrete target Hb level
affords clarity and simplicity, but is almost universally impossible to
achieve, renders implementation as a performance measure difficult, and
discourages flexibility in using tradeoff decision making to meet the
needs and preferences of individual patients.
Similarly, the Work Group considered, but rejected, identifying a
target Hb level bounded by narrow upper and lower values (eg, 11.0 to
12.0 g/dL). Such a target affords neither clarity nor simplicity, is
possible to achieve in only a minority of patients,111-113
discourages flexibility in managing individual patients, and likely
promotes cycling of Hb results greater than and less than the target.114
The Work Group chose to define a broad therapeutic range of Hb levels
bounded by a discrete lower threshold above which Hb level should be
maintained and a upper threshold above which Hb should not be routinely maintained. This approach makes clear to the practitioner that no patient should be maintained intentionally
at less than the lower threshold, and patients should not be routinely
maintained at greater than the upper threshold. We chose 11.0 g/dL as
the lower Hb level threshold. Hb values at or near 11.0 g/dL comprise
an approximate watershed, defining the upper Hb targets of early RCTs
and the lower targets of more recent trials (Fig 15).
Thus, evidence to support the lower limit of Hb statement draws on the
full range of RCTs available and the full range of Hb targets examined.
This evidence supports the conclusion that patients treated to a Hb
target greater than 11.0 g/dL likely will experience measurable QOL
benefits with little or no increase in AEs compared with treatment at
lower Hb levels.
Hb values greater than 11 g/dL are readily
achievable. Greater than 80% of prevalent patients with HD-CKD in the
United States currently demonstrate Hb levels of 11.0 g/dL or greater.28
Among patients with a Hb level less than 11.0 g/dL, greater than 99.6%
achieve Hb values of 11.0 g/dL or greater within 6 months when
prescribed sufficient ESA. Thus, the guideline as stated can be
implemented readily in clinical practice and provides a rational
foundation for developing clinical performance measures for anemia
management.
In the opinion of the Work Group, there
is insufficient evidence to recommend routinely maintaining Hb levels
at 13.0 g/dL or greater in ESA-treated patients.
Evidence reviewed in the foregoing rationale supports the conclusion
that QOL benefits associated with treating to a higher Hb target extend
throughout the full range of Hb targets examined to date, spanning 6 to
16 g/dL. However, the same evidence suggests that treating to Hb
targets greater than 13 g/dL may increase the risk for serious AEs. The
only trial that had sufficient power to examine safety assigned
patients to a target Hb level of 14.0 g/dL in the higher-target
treatment arms108
and compared outcomes with those seen in patients assigned to a lower
Hb target (10.0 g/dL). That trial was discontinued for safety concerns.
In a second trial, patients assigned to a higher Hb target (13.5 to
14.5 g/dL) showed an increased incidence of cerebrovascular AEs
compared with those assigned to a lower target (9.5 to 11.5 g/dL).109
Two additional trials compared the efficacy and safety of Hb targets in
patients with ND-CKD, Cardiovascular Risk Reduction by Early Anemia
Treatment With Epoetin Beta Trial (CREATE; Hb level of 10.5 to 11.5
versus 13.0 to 15.0 g/dL)115 and Correction of Hemoglobin and Outcomes in Renal Insufficiency (CHOIR; Hb level of 11.3 versus 13.5 g/dL).115a
The CREATE trial has been completed, whereas the CHOIR trial has been
terminated early by decision of the trial safety committee. Results
from either trial are not published at the time of this writing.
In short, efforts to gain QOL benefits by treating to Hb targets of 13
g/dL or greater are countered by increased risk for life-threatening or
disabling AEs. The statement that There is insufficient evidence to recommend routinely maintaining Hb levels at 13.0 g/dL or greater
recognizes the concern that, for most patients, the known risks
outweigh the known benefits of treating to higher Hb level. The phrase insufficient evidence reflects the finding that published results from comparative trials large enough to examine safety are relatively limited.
Moreover, the word routinely
reflects the crucial limitations of currently available evidence and
the need for further investigation. For example, no clinical trial
conducted to date has examined target Hb safety and efficacy in
selective subgroups that may especially benefit from higher Hb targets,
including, for example, patients who live at high altitude, are
employed, have pulmonary disease, have high levels of physical
performance, or undergo daily or nocturnal HD. In the absence of
information in these and other potential subgroups, medical decision
making requires weighing known risks and benefits in mixed-study
populations against unknown risks and unknown benefits in individual
patients.
The statement Routinely maintaining patients at Hb levels of 13 g/dL or greater
makes it clear that the recommended upper limit of Hb level applies
only to patients considered for maintaining a Hb level at 13 g/dL or
greater as a treatment target, not patients for whom Hb level is likely
to transiently equal or exceed 13 g/dL while undergoing treatment for a
lower Hb target, and not patients who do not require ESA therapy. Given
the biological variation in Hb levels in patients with HD-CKD, if the
intent of treatment is to maintain Hb levels at 11.0 g/dL or greater, a
sizeable fraction of patients will necessarily show Hb values
transiently at 13 g/dL or greater. Judicious ESA dose adjustments
appear sufficient to correct Hb values above intended targets within 3
to 6 months.112
LIMITATIONS
Limits of Available RCTs
The strength
of an RCT is to study a specific question by studying the efficacy of
an intervention on specific outcomes in a well-defined population.
Findings from RCTs thus are often limited in their generalizability to
diverse populations or to questions related to effectiveness of
combinations of interventions on combinations of patient outcomes.
These latter questions are what guidelines attempt to address. For
example, consider a hypothetical study designed to compare the effect
of 3 Hb treatment targets: Hb level less than 9 g/dL, Hb level of 9 to
12 g/dL, and Hb level greater than 12 g/dL. Patient populations should
be separated into patients with or without CVD and: (1) ND-CKD, (2)
HD-CKD, (3) PD-CKD, or (4) transplant-associated CKD. Each discreet Hb
target, CVD status, and each CKD category deserves scrutiny. However,
this is unfeasible: the hypothetical RCT would collapse under the
weight of a 3 × 2 × 4 (24-group) study design.
Imagine instead constructing a 24-cell matrix to represent all possible
combinations of Hb targets, CVD status, and CKD categories that we wish
to examine. If we added all the results from all available RCTs to the
specific cells addressed by the available RCTs, we would have to leave
the vast majority of cells empty at this writing. Given the small
number of RCTs and the small size of most of the available RCTs, and
given the potential differences between target populations, filling in
empty cells by extending results from the few completed cells seems
unwarranted and likely to involve substantial error.
Another critical limitation of the evidence, as discussed, is the lack
of information about the comparative safety and efficacy of lower
versus higher Hb targets in selected subgroups of patients that may
especially benefit from higher Hb targets.
QOL/QOL Trade-Off
QOL, like quantity
of life, is a hard patient outcome. Available evidence confirms that
setting Hb targets for individual patients requires consideration of
the potential trade-off between QOL and survival, MI, cerebrovascular
event, or access thrombosis. However, although we know that risk
assessment varies widely by culture, personal values, level of
education, and level of medical literacy, we lack information on how to
assist patients with CKD to interpret risk, judge risk-benefit
trade-offs, and express informed preferences. Nevertheless, in everyday
medical decision making, it is important to incorporate informed
patient preference.116
Differences in Treatment Strategies Used To Achieve Defined Hb Targets
The duration of anemia before initiating ESA therapy and the size and
rate of Hb level correction each plausibly contributes to risk,
benefit, or both in anemia therapy. Information on each would be
directly helpful to clinicians.
Similarly, it is possible that the risks and benefits associated with a target Hb level depend in part on which therapeutic agents were used in the treatment arm. All available hard-outcome trials to date have used both ESAs and iron agents together in patients assigned to both treatment and control arms. No trial using ESA alone, iron alone, or noniron adjuvants alone has been adequately designed and powered to confirm that similar Hb targets afford similar risks and benefits regardless of how they are attained. The possibility that each potential class of agents, including ESAs, oral iron, parenteral iron, and non-iron adjuvants, shows differing patient outcomes for equivalent Hb outcomes has not been explored.
| © 2006 National Kidney Foundation, Inc. |