16.1 Serum levels of calcium, phosphorus, total CO2 and plasma intact PTH should be monitored following kidney transplantation. (OPINION)
16.1a The frequency of these measurements should be based on the time following transplantation, as shown in Table 33. (OPINION)
16.2 During the first week after kidney transplantation, serum levels of phosphorus should be measured daily. Kidney transplant recipients who develop persistently low levels of serum phosphate (<2.5 mg/dL [0.81 mmol/L]) should be treated with phosphate supplementation. (OPINION)
16.3 To minimize bone mass loss and osteonecrosis, the immunosuppressive regimen should be adjusted to the lowest effective dose of glucocorticoids. (EVIDENCE)
16.4 Kidney transplant recipients should have bone mineral density (BMD) measured by dual energy X-ray absorptiometry (DEXA) to assess the presence or development of osteoporosis. (OPINION)
16.4a DEXA scans should be obtained at time of transplant, 1 year, and 2 years post-transplant. (OPINION)
16.4b If BMD t-score is equal to or less than -2 at the time of the transplant or at subsequent evaluations, therapy with parenteral amino-bisphosphonates should be considered. (OPINION)
16.5 Treatment of disturbances in bone and mineral metabolism is determined by the level of kidney function in the transplant recipient as provided in Guidelines 1 through 15 for CKD patients. (OPINION)
Kidney transplantation is an effective modality for therapy of end-stage kidney disease; while successful kidney transplantation reverses many problems of uremia which are not corrected by dialysis therapy, osteodystrophy (disorders of bone remodeling and modeling) persists. Ninety to one hundred percent of kidney transplant patients have histological evidence of osteodystrophy525,526 and osteopenia (reduction of bone mass)527 following transplantation. Most importantly, the loss of bone mass in the early post-transplant period produces osteopenia and osteoporosis (bone mass reduction more than 2 standard deviations below young adult peak bone mass according to the World Health Organization).528 In addition, a sizable number of patients suffer from avascular necrosis (AVN), usually in the first 2 years following transplantation.527
Hypercalcemia
Hypercalcemia following kidney transplantation is common and is usually due to hyperparathyroidism that persists from the preceding period of chronic kidney disease. Restoration of kidney function partially reverses the resistance to the calcemic action of PTH and restores calcitriol production, with consequent hypercalcemia from increased intestinal calcium absorption and the effects of PTH on kidney calcium transport and bone turnover. The hypercalcemia generally resolves as the parathyroid gland hypertrophy is reversed in the presence of sufficient kidney function. However, in 1% to 5% of transplant recipients, abnormal PTH secretion persists, causing hypercalcemia that may require parathyroidectomy.
Hypophosphatemia
Hypophosphatemia occurs in 50% to 80% of patients in the first 3 months after kidney transplantation and is due to hyperphosphaturia.529-544 By 3 months after transplant, 0% to 26% of patients will be hypophosphatemic541-544 and by 1 year after transplant, it can be expected that <5% of patients will still be hypophosphatemic.542-544 The hyperphosphaturia is multifactorial in origin and is related to persistent hyperparathyroidism,156,529,537,538,541,545-551 immunosuppressive and diuretic drugs, reduced intestinal absorption of phosphorus,530,534,535,537,540,552-554 and possibly the existence of a phosphaturic substance, like phosphatonin, which is present in the serum of kidney transplant patients.530,539,545,546,555,556 This phosphaturic substance promotes phosphate loss via the nephron, independent of parathyroid hormone (PTH). In patients without CKD or kidney transplant, hypophosphatemia can lead to extrarenal complications such as osteopenia, osteomalacia,557-561 rhabdomyolysis, impaired cardiac contractility, cardiac arrhythmias, respiratory failure, neurological complications, hemolytic anemia, and leukocyte dysfunction.562-565 For these reasons, it is important to determine whether treatment of hypophosphatemia should be undertaken in kidney transplant patients.
Metabolic Acidosis
Metabolic acidosis following kidney transplantation may be present due to a variety of causes. Skeletal buffering of excess protons contributes to the abnormalities in calcium and phosphorus metabolism and the disorders of bone remodeling seen following kidney transplantation. Correction of the acidosis may assist in the general resolution of the electrolyte abnormalities and a lessening of the forces producing post-transplant osteoporosis.
Hyperparathyroidism
After successful kidney transplant, biochemical evidence of hyperparathyroidism can persist in many patients.557,566-572 Persistent hyperparathyroidism has negative effects upon bone mineralization and may also worsen skeletal complications such as osteopenia and rates of bone fracture.557,569 Persistent hyperparathyroidism is potentially a risk factor for hypercalcemia, hypophosphatemia, worsening of bone disease, and possibly acute tubular necrosis after kidney transplant.571,572 Because this increased risk may be injurious to patients, the Work Group felt that a review of this area and treatment recommendations were in order.
Hypercalcemia
With the restoration of kidney function following transplantation, the parathyroid glands that may have hypertrophied during the period of CKD must involute. Until this is complete, patients are at risk for hypercalcemia due to hyperparathyroidism. Failure of the parathyroid glands to involute sufficiently to decrease PTH secretion to levels appropriate for calcium homeostasis in the post-transplant period is an indication for parathyroidectomy. This is observed in about 1%-5% of transplant recipients.
Hypophosphatemia
Associated with restoration of kidney function following transplantation, high rates of urinary phosphate excretion are observed that often lead to the development of hypophosphatemia. The cause of the phosphaturia is a reduction in the tubular maximum (Tm) for phosphate in the proximal tubule. Multiple factors contribute to this dysregulation of tubular phosphate transport which may in part be due to decreased levels of a type II sodium-dependent phosphate transport protein (NaPi2) in the brush-border membrane. The regulatory influences that would produce such a situation include PTH, glucocorticoids, and phosphatonin. The latter is a newly discovered hormonal regulator of phosphate transport that is responsible for hypophosphatemia in cases of oncogenic osteomalacia and autosomal dominant hypophosphatemic rickets (ADHR). Phosphatonin is normally processed by subtilisin-like proprotein convertases and degraded by endopeptidases including the product of the gene PHEX that is responsible for the disease, X-linked hypophosphatemia. In this genetic osteomalacia, mutations in PHEX lead to failure of phosphatonin degradation and severe hypophosphatemia due to decreased levels and inhibition of NaPi2 activity in the proximal tubule. Similarly, following kidney transplantation high levels of phosphatonin may occur and contribute to the hypophosphatemia observed.
Hyperparathyroidism
Biochemical evidence of hyperparathyroidism may persist in a large number of patients after successful kidney transplant.547-551,573-579 The increases in PTH are believed to be due to a large glandular mass of parathyroid cells that develops during CKD and persists after kidney transplant.547-551,557,566-579 Kidney transplantation can have beneficial effects upon hyperparathyroidism by reversal of hyperphosphatemia and production of 1,25-dihydroxyvitamin D3 by the renal allograft.156,542,580,581 PTH levels tend to decline over time as the hyperplastic parathyroid glands undergo involution.582,583
There are many longitudinal studies of the behavior of PTH levels after kidney transplantation. The PTH levels decline rapidly after transplant, then decline at a slower rate,547-551,573-579,582,583 generally decreasing by 50% within 14 days of transplant. Within approximately 3 to 6 months after successful transplant, it can be anticipated that 50% of patients with serum creatinine <2.0 mg/dL (177 mmol/L) will have normal levels of intact PTH.547,579,584 Most reports indicate that intact PTH levels usually return to near normal by 1 year after transplant.542,547-550,579-581,584-590 However, PTH levels can remain above normal, presumably due to persistent parathyroid hyperplasia, as evidenced by other reports. One group found 50% of patients with PTH levels above normal at 24 months after transplant.543 In a long-term study, 21% of transplant patients had significant biochemical hyperparathyroidism up to 15 years after transplantation,591 but this presumably relates to low levels of kidney function.156,575
Persistent elevations in the levels of PTH have been associated with a longer duration of dialysis prior to kidney transplant,592 along with high levels of PTH156 and high values of serum calcium at the time of transplantation.568,593 There are conflicting studies regarding the importance of vitamin D receptor genotype upon the persistence of hyperparathyroidism after transplant. One study found that patients with BB genotype had lower PTH levels post-transplant,577 but another noted lower PTH levels in patients with bb genotype for vitamin D receptor.578
Some kidney transplant patients will require parathyroidectomy to alleviate hyperparathyroidism. In general, most studies indicate a prevalence rate of approximately 5% for kidney transplant patients who will require parathyroid surgery, but the prevalence rate varies from 1%-20% among different transplant centers.575,576,584,587,592,594-599 Parathyroid surgery may be indicated for persistent hypercalcemia in transplant patients (particularly if serum calcium is =11.5 mg/dL [2.87 mmol/L]).595,596 Other indications for parathyroidectomy after kidney transplant include calciphylaxis, rapidly worsening vascular calcification, symptomatic hyperparathyroid bone disease, and the development of spontaneous fractures in the presence of hyperparathyroidism.575,576,584,587,588,592,594-599 One group advocates waiting at least 1 year post-transplant to see if there is spontaneous regression of hyperparathyroidism.597 There is no consensus on the proper parathyroidectomy operation that should be performed, with some authors advocating subtotal parathyroidectomy and others advocating total parathyroidectomy with autotransplantation of parathyroid tissue.575,576,584,587,592,594-596
Previous studies comparing the predictive value of C-terminal PTH assays have shown that the results are not always indicative of an elevated N-terminal PTH assay.549,550,557,558,578,581,600-602 PTH levels are not always indicative of the rate of bone turnover in kidney transplant patients.557,558,581,600-602 In fact, PTH levels may have limited value for the assessment of bone turnover in kidney transplant patients. In a study of 4 patients with PTH levels >100 pg/mL (11.0 pmol/L), 3 had normal bone turnover.601 Several studies that have used bone biopsies to document bone turnover have shown that kidney transplant patients with osteopenia may have several bone histological patterns that are not due to hyperparathyroidism. These bone biopsy findings describe a low-turnover bone lesion557,600 similar to osteoporosis, persistent hyperparathyroidism,551,578,602,603 and even osteomalacia.581 Previous studies have indicated a relatively high prevalence of increased bone turnover in kidney transplant patients. However, a more recent cross-sectional study of bone biopsy findings in kidney transplant patients, with an average of 5.4 years after transplant, noted low bone turnover in 46% of patients.558 Mineralization lag time was prolonged in approximately 88% of patients undergoing bone biopsy. PTH levels were not always indicative of the rate of bone turnover.
Osteopenia/Osteoporosis and Fractures
Osteopenia is nearly a uniform finding in the late post-transplant period (longer than 2 years post-transplant).525,604 Older studies underestimated the severity of osteopenia, since they were performed when osteopenia was not generally present at the time of transplant.525,526 Pretransplant osteitis fibrosa and pre-existing osteodystrophy, associated with high bone turnover rates or osteomalacia, were the prevalent forms of bone disease at the time of these studies, and osteopenia was not commonly associated with chronic dialysis therapy.605 In recent studies, osteopenia due—in part—to the adynamic bone disorder has become more prevalent in CKD, and represents a significant component of bone disease at the time of kidney transplantation.23,559
High rates of trabecular bone fracture complicate solid organ transplantation (kidney, liver, heart, lung, and pancreas). Vertebral bodies, ribs, and hips are the sites most often affected. These fractures represent major stumbling blocks to the post-transplant rehabilitation that would otherwise be expected. Fractures add tremendously to the costs of health care for these patients. The causes of the increased fracture risk in the kidney transplant population is an osteodystrophy associated with rapid reductions in bone mass during the first 2 years post-transplant. Kidney transplantation is characterized by various pretransplant osteodystrophies, but the main pathophysiological issue is a rapid loss of bone mass in the early post-transplant period. The cause of this early post-transplant bone loss is unknown, and its pathophysiological mechanism has not been accurately characterized. However, there is a growing consensus that—similar to other clinical situations in which glucocorticoids are used therapeutically—their use is the major factor producing decreased osteoblast function and loss of bone mass. Further worsening of osteopenia occurs up to 2 years post-transplant, and fractures occur during the entire post-transplant period. Transplant recipients should be monitored for this rapid loss of bone mass. DEXA is the clinical standard for measurement of BMD,528 and patients should be monitored for changes in their bone mass on a regular basis following kidney transplantation. If osteoporosis is identified by changes in BMD, therapy should be initiated.
The nature of osteodystrophy prior to transplantation has changed in the last decade. As a result, more patients are osteopenic at the time of transplant due to the adynamic bone disorder. At the same time, the rapid bone loss that occurs following transplantation has not been prevented. Therefore, post-transplant fracture rates remain high, and post-transplant osteodystrophy (which is often an osteoporosis) with fractures and osteonecrosis are major causes of morbidity associated with long-term post-transplant survival.
High rates of fracture also complicate cardiac, liver, pancreas, and lung transplantation to even a greater degree than in kidney transplantation. The chronic illnesses leading to these organ transplants often produce osteopenia secondary to inactivity or immobilization. Associated with the immediate post-transplant period, there is a very significant bone loss resulting in a major reduction in BMD within the first 6 months following the operation. High rates of fracture complicate the first 2 years of the post-transplant period. The cause of the rapid loss of bone mass associated with organ transplantation has been attributed to the use of glucocorticoid and immunosuppressive therapy as contributing factors.606-611
Hypophosphatemia
The Work Group did not identify any double-blind, randomized, placebo-controlled trials (highest-quality evidence) regarding treatment of hypophosphatemia in kidney transplant patients. There were 2 prospective trials on the effect of phosphate supplementation. One study reported a single group pre-treatment versus post-treatment156; this study included transplant patients with serum phosphorus <3.5 mg/dL. This was a very short-term study of only 15 days’ duration. These patients had been transplanted for an average of 41 months, and they had normal kidney function. Patients received oral neutral phosphate in a dosage of 750 mg BID, and they were restudied after 2 weeks of therapy. Phosphate supplements tended to decrease serum calcium, increase serum phosphorus, increase levels of PTH, and decrease serum levels of 1,25-dihydroxyvitamin D. Very few patients increased their serum phosphorus level to 4.5 mg/dL (1.45 mmol/L). The authors contended that phosphate administration after kidney transplantation may worsen hyperparathyroidism, and that if phosphate supplements are used, concomitant calcitriol administration may be of value to maintain calcitriol levels and avoid worsening of hyperparathyroidism.
Another randomized, controlled trial studied transplant patients who were hypophosphatemic after transplant (serum phosphorus 0.93 to 2.34 mg/dL [0.30 to 0.76 mmol/L]).532 Patients were randomized to receive either neutral sodium phosphate or sodium chloride for 12 weeks. Within 2 weeks, the phosphorus-supplemented group increased serum phosphorus levels, but by week 12 of the study, both groups had average phosphorus levels of approximately 2.5 mg/dL (0.81 mmol/L). At the beginning of this study, muscle phosphorus content was below normal in both groups. By week 12 of the study, the phosphate-supplemented group had slightly higher muscle ATP content than the sodium chloride-treated group. The phosphate-treated group also tended to have higher levels of plasma bicarbonate. PTH levels declined in both groups.
A third study treated 10 kidney transplant patients with oral calcium carbonate (1,000 mg/day) and either dihydrotachysterol (0.37 mg/day) or oral calcitriol (0.60 µg/day).540 All patients had serum creatinine levels <2.0 mg/dL (177 mmol/L), had been transplanted for 0 to 44 months (average, 14 months), and all patients were already using oral phosphate supplements. With calcium and vitamin D analogs, the phosphate supplement dosage decreased from 8.0 g/day to 4.6 g/day and the serum phosphorus remained in the range of 2.7 to 3.1 mg/dL (0.87 to 1.00 mmol/L). Serum calcium increased from 9.3 mg/dL (2.32 mmol/L) to 10.0 mg/dL (2.50 mmol/L) and PTH values declined by 30% to 40% from initial values. The renal fractional excretion of phosphorus declined.
The Work Group also recognizes that, in patients without CKD or kidney transplantation, it is common practice to provide oral phosphate supplements when the serum phosphorus declines below 1.0 mg/dL (0.32 mmol/L).562-565 A serum phosphorus level <1.5 mg/dL (0.48 mmol/L) is commonly defined as severe hypophosphatemia and phosphate supplementation with either oral or intravenous compounds would be recommended in non-CKD patients.562-565
Upon review of the literature, it appears that there is no clear consensus as to the level of serum phosphorus that should prompt phosphate supplementation in CKD patients with kidney transplant. The Work Group found that the overall literature regarding phosphate supplementation was not entirely clear. A recent review of this subject has suggested that phosphate supplementation is indicted if the serum phosphorus level declines below 2.5 mg/dL (0.81 mmol/L) in patients with kidney transplant.612
Based upon review of the available literature, the Work Group recommends that kidney transplant patients with serum phosphorus levels <1.5 mg/dL (0.48 mmol/L) should receive oral phosphate supplements to achieve a serum phosphorus level of 2.5 to 4.5 mg/dL (0.81 to 1.45 mmol/L). CKD kidney transplant patients with serum phosphorus levels of 1.6 to 2.5 mg/dL (0.52 to 0.81 mmol/L) may often require oral phosphate supplements with a desired serum phosphorus target range of 2.5 to 4.5 mg/dL (0.81 to 1.45 mmol/L). The Work Group further recommends that, when phosphate supplements are administered, serum phosphorus and serum calcium levels should be measured at least weekly. If serum phosphorus levels exceed 4.5 mg/dL (0.81 mmol/L), then the dosage of phosphate supplements should be decreased.
PTH levels should be determined and the patients should be examined for evidence of persistent hyperparathyroidism if oral phosphate supplements are required to maintain serum phosphorus levels >2.5 mg/dL (0.81 mmol/L) more than 3 months after kidney transplant. These patients may require the use of oral calcium supplements, and possible coadministration of vitamin D analogs.
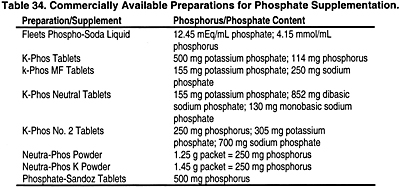
Beyond 3 months after kidney transplant, the levels of PTH, calcium, and phosphorus should be measured at a frequency that is commensurate with the level of GFR (see Guideline 1). Table 34 lists some of the available preparations for phosphate supplementation.
Osteopenia/Avascular Necrosis and Fractures
The nature of the osteodystrophy following kidney transplantation is not well established. Histological studies have been reported in fewer than 200 patients. An Italian study525 identified 3 histological patterns: low bone turnover lesions similar to osteoporosis; persistent osteitis fibrosa; and osteomalacia. Others525 described persistent osteodystrophy and osteopenia in a high percentage of transplant patients at 1 year post-transplant, but failed to establish the relationship between histomorphometry and clinical symptomatology. Lesions with a very low rate of bone formation have been described in patients with avascular necrosis.527 Recent studies have described a high prevalence of low bone turnover, osteoporosis, and osteomalacia following kidney transplantation.558 Studies of BMD have established a rapid decrease in bone mass following kidney transplantation with a nadir around 6 months that tends to slow or recover by 1 year.557 The osteodystrophy of CKD has evolved significantly since some of the above studies were performed. The prevalence of osteomalacia has diminished. A new form of osteodystrophy, adynamic bone disorder (also known as "adynamic bone disease" or "low-turnover bone disease") independent of aluminum intoxication, has become common in the patients with Stage 5 CKD.613 The impact of kidney transplantation on this form of pretransplant osteodystrophy has not been carefully elucidated.
A study of 20 patients with mild osteitis fibrosa prior to transplant demonstrated that all the patients sustained a major post-transplant loss of bone mass by 6 months.557 The loss of bone mass was characterized by a marked decrease in mineral apposition and bone formation rate, resulting in major prolongation of mineralization lag time and formation periods while there was a reduction of the elevated pretransplant rate of bone resorption to normal. This correlated with a decrease in bone densitometry assessed by dual-photon absorptiometry and a persistent decrease in BMD at 18 months, in addition to the reductions seen at 6 months. Thus, these patients developed osteoporosis and an adynamic bone disorder but the course of patients with adynamic bone disorders prior to transplant was unknown. Others have confirmed these findings, but with the added finding that osteomalacia was present more often than expected.558 A study using DEXA demonstrated, in 34 kidney transplant recipients, that bone mineral content and BMD were decreased 7% and 5%, respectively, by 5 months.559 These authors also noted a 3% reduction in spine BMD at 3 months which was progressive to 5 months post-transplant. Large cross-sectional studies of BMD post-transplant demonstrate that bone loss continues for the first 2 years, reaching reductions of 10% at 12 months and 16% at 24 months compared to the normal population.604
The clinical impact of the osteodystrophy and bone loss is a marked increase in the fracture rate associated with kidney transplantation from 0.009 fractures per patient per year pre-transplant to 0.032 post-transplant.614 Peripheral bone fractures affected 10% to 15% of kidney transplant recipients, and a similar percentage of the patients sustained vertebral fractures.
Persistent hyperparathyroidism is a factor that could play a role in the osteopenia that develops after kidney transplantation. Osteopenia has been described in patients with primary hyperparathyroidism.615 It is unclear whether persistent hyperparathyroidism is a major factor contributing to early bone loss before involution of hypertrophic parathyroid glands.530 Osteitis fibrosa is less frequent but still observed in post-kidney-transplant bone biopsies.526,557,558,582 Recent studies demonstrate that the severity of pretransplant hyperparathyroidism predicted the magnitude of the change in vertebral bone mineral density.616
Glucocorticoid (eg, prednisone) therapy is a major factor in the development of osteopenia. Glucocorticoids have been shown to have a variety of effects on calcium metabolism which are likely to decrease bone mass.617,618 These include increases in urinary calcium and phosphate excretion, direct and indirect (via interference with vitamin D metabolism) inhibition of intestinal calcium absorption, and suppression of osteoblast and enhancement of osteoclast function.618-621 Data from noninvasive techniques document a suppression of serum osteocalcin and urinary hydroxyproline during a short course (12 weeks) of high-dose prednisone therapy (mean dose, 34 mg/day) in patients with Graves’ ophthalmopathy.619,622 Other studies have concluded that cumulative and maintenance doses of prednisone correlated inversely with bone volume and bone turnover following kidney transplantation,558 but this remains a controversial issue.
Using the methodology for study selection described earlier in these Guidelines, the Work Group identified 5 studies examining 302 patients that studied the relationship between immunosuppressant-steroid combinations and bone mineral density (BMD).561,623-626 Four studies used DEXA as the method for measuring BMD, while 1624 used dual-photon absorptiometry. Four studies were controlled trials,561,623,625,626 while 1 was a case-control study.624 Three of the five studies were retrospective561,624,625; the other 2 were prospective, randomized, controlled trials.623,626 These studies all examined different patient populations and different interventions, and this did not allow the Work group to do any specific synthesis or meta-analysis of the results. The evidence review comprised a qualitative literature review of these studies.
Three of the controlled trials561,625,626 compared a variety of immunosuppressant and steroid combinations. No new studies looked at the same drug combinations. In the only prospective trial,626 the authors compared single, double, and triple therapy. They found that cyclosporine monotherapy led to improved BMD compared to cyclosporine plus prednisone, or cyclosporine plus prednisone and azathioprine. This group did not note any statistically significant differences in BMD between the double and triple therapy groups over 18 months.626 One retrospective study found no differences in BMD between groups receiving cyclosporine versus azathioprine plus prednisone in the 7 to 15 years after transplant.625 Another retrospective study compared patients who had received <10 mg of prednisone per day to those receiving >10 mg prednisone per day.561 At the end of 1 year, DEXA measurements of BMD had improved slightly in the low-prednisone group versus the high-prednisone group, and the inter-group difference was statistically significant (d = 1.409, 95% CI, 0.92 to 1.02, Hedges’ d test for standard effect sizes).
In a study conducted on children, using a controlled design, the authors calculated the change in BMD over a 1-year period when patients were switched from prednisone to deflazacort, or continued taking prednisone, both in conjunction with cyclosporine and azathioprine or mycophenolate.623 Thus, although the only intended inter-group difference was the type of glucocorticoid given, the use of mycophenolate rather than azathioprine in 5 patients in the prednisone group may have affected results. Both groups showed declining BMD over the 1-year period. No statistically significant differences were found in BMD measurements between the 2 groups over the 1-year period of the study.
Immunosuppressive therapy may have an impact on bone remodeling. Transplant recipients routinely receive cyclosporine A or tacrolimus. These immunosuppressive agents are used in high doses in heart and liver transplantation, and may be associated with worse osteopenia than is observed following kidney transplantation, in which lower doses of these agents are generally used. Cyclosporine’s major effect on bone cells in vitro is to inhibit osteoclastic bone resorption,627 possibly by decreasing the formation of mature osteoclasts.628 Effects of cyclosporine A in vivo appear to differ from its actions in vitro. The effects of cyclosporine A on bone modeling have been studied in rats.629-631 This series of studies demonstrated that cyclosporine A produced osteopenia through stimulating resorption, and that elevated osteocalcin levels were related to increased bone turnover. There are major difficulties translating these results to the human post-transplant situation, but they indicate a major need to carefully analyze post-transplant osteodystrophy mechanistically for combined effects of corticosteroids and cyclosporine A. They appear to demonstrate one reason that bone resorption is higher than expected for the major decrease in bone formation that occurs following transplantation. On the other hand, a study of cyclosporine A in kidney transplant patients suggests that it reduces the incidence of avascular necrosis (AVN) by permitting a lower dose of steroids.632 However, this study was flawed by the use of historical controls. The effect of cyclosporine A to increase osteocalcin levels, which has been observed in post-transplant osteodystrophy in general, may be additive to the low-turnover osteodystrophy produced by glucocorticoids. Also, the nephrotoxic effects of cyclosporine and tacrolimus may lead to secondary hyperparathyroidism when glomerular filtration rates drop to the range of 40% to 50% of expected.633
Avascular Necrosis (AVN)
AVN is a well-recognized complication of kidney transplantation. It usually begins at the weight-bearing surface of the femoral head with collapse of surface bone and cartilage. With time, the area of collapse spreads to involve a large proportion of the femoral head. Pain is severe and patients are often unable to bear weight on the involved hip. Other weight-bearing joints are also frequently involved.634 Speculations about the mechanism for this development mostly focus on the observation that fat cells are present in increased numbers and intraosseous pressure is high. This has led to the theory that the proliferation of fat cells causes the high intraosseous pressure with subsequent interference with perfusion of the bone.634 Glucocorticoid dosage635 and prior dialysis527 appear to be important in the development of AVN. Patients on maintenance dialysis for longer duration prior to transplantation are more likely to develop AVN.527 No mention is made in this report of the particular type of bone disease that these patients had, though, with longer duration of dialysis, both osteitis fibrosa and osteomalacia are increased in frequency.18,636
Using the methodology described previously, the Work Group identified 7 studies dealing with the relationship between immunosuppressant and glucocorticoid therapies, and avascular necrosis.632,637-642 All 7 studies varied either the dosage of glucocorticoids or glucocorticoid-immunosuppressant combination. These 7 studies enrolled a total of 1,471 patients. Most of the patients were from 3 retrospective trials632,641,642; thus, the bulk of patient data comes from lower-quality studies. Five controlled studies compared high- versus low-dose glucocorticoids.638-642 In all 5 of these studies, the glucocorticoids were given in conjunction with azathioprine. Both prednisone and prednisolone were used in these studies, but no distinction was made for the purpose of this analysis. These 5 studies reported different dosage of glucocorticoids, but all compared "high dose" versus "low dose." Such data did not allow the Work Group to determine the "best" dose of glucocorticoid.
Fig 16. Effect sizes and 95% CI of RCTs and retrospective studies reporting incidence of necrosis.
Based on these 5 studies, a meta-analysis indicated that high-dose glucocorticoids plus azathioprine resulted in significantly more cases of avascular necrosis than did an azathioprine plus low-dose glucocorticoid combination (P < 0.001, Hedges’ d test, odds ratio calculated according to the method of Hasselblad and Hedges38). The results are graphically depicted in Fig 16 . These results were then confirmed using the log-odds ratio method (odds ratio = 2.74, 95% CI, 1.69 to 4.44, P < 0.001).
To further facilitate interpretation of these results, a statistical analysis in the form of a binomial effect size display was performed. This analysis indicated that patients taking high-dose glucocorticoids have at least a 1.5-fold greater risk of avascular necrosis than those taking low-dose glucocorticoids.
The Work Group also noted that, of the studies presented, cyclosporine was part of the treatment regimen in only 2 studies.632, 637 Thus, caution must be used in applying these observations to the currently used immunosuppressant regimens, which often include cyclosporine or other calcineurin inhibitors.
Therapy for Post-Transplant Osteodystrophy
Therapy with replacement of gonadal steroid hormones for estrogen-deficient and testosterone-deficient patients should be seriously considered following transplantation. These are effective therapies for increasing bone mass in patients with osteoporosis, although their use following transplantation has not been adequately studied. However, patients with kidney disease often have gonadal hormone deficiencies that are untreated.
A major group of drugs to consider in the therapy of post-transplant bone loss is the bisphosphonates. These drugs have the unique capacity to bind to bone surfaces for prolonged periods.643 They primarily inhibit osteoclastic bone resorption. Etidronate, the bisphosphonate available in the United States until the release of pamidronate and alendronate, unfortunately inhibits bone formation as well as resorption, when given continuously. Recent reports document that intermittent administration of etidronate maintains the antiresorptive effect. In addition, these reports644 delineate a possible benefit in terms of bone mass and fracture prevention in osteoporosis. Etidronate therapy post-transplant has been a failure both in the prevention of BMD loss, and in preventing fractures.645,646
Aminohydroxypropylidene (APD), or pamidronate, has been used in patients receiving steroids, and bone mass was well preserved.647 There are limited data on the use of APD in disorders other than Paget’s disease. In Paget’s disease, the effects of APD following a single injection are sustained for months. The use of APD in kidney and heart transplant patients with BMD as the endpoint have recently been reported with promising preliminary results in the prevention of BMD changes at 6 months and 1 year.648,649 A small, randomized study of transplant recipients treated in the early post-transplant period648 demonstrated that the control subjects lost 6.4% of BMD at 12 months compared to no loss of BMD in the pamidronate group.646
Alendronate has recently been carefully studied in osteoporosis.650 Women receiving alendronate experienced progressive increases in BMD at all skeletal sites including the spine, femoral neck, and trochanter. In addition, in 1 study, the drug resulted in a 2.5% increase in total body BMD. Alendronate produced a 48% reduction in the proportion of women with new vertebral fractures and a reduction in the loss of height in the vertebral bodies. It appears that alendronate may progressively increase bone mass in the spine, hip, and total body, and reduce the incidence of vertebral fractures in patients with osteoporosis. The drawback of alendronate therapy is that it is only available in an oral form in the United States, and it has to be taken very carefully to avoid esophageal irritation.651
Another inhibitor of resorption, calcitonin, holds promise. This naturally occurring hormone has been studied extensively. With documented efficacy in the treatment of Paget’s disease, malignant osteolysis, and high-turnover osteoporosis,652 this agent has also been shown to protect bone mass in conventional osteoporosis653 A recent report654 even describes long-term effects (1 year) after short-term therapy (6 to 8 weeks). Finally, clinical trials with calcitonin glucocorticoid-treated patients have shown promise.655 Calcitonin acts by inhibiting osteoclast action,656 although others have presented in vitro data showing that calcitonin may also stimulate osteoblast function.657 Until recently, calcitonin’s use has been limited by the need to give it by injection. The development of the nasal route of administration has considerably improved patient acceptance.658 In addition, side-effects and toxicity, known to be low, are even less by this route than by injection. Bioavailability is also excellent with the intranasal preparation, showing 50% to 100% of the availability of the intravenous route.659 Animal studies have documented low toxicity and demonstrated a bone effect limited to suppression of osteoclastic activity without deleterious effects on bone formation.660 A small, randomized trial of nasal calcitonin (200 IU) compared to oral clodronate (800 mg/dL; a bisphosphonate not available in the United States) in patients in the late post-transplant period, demonstrated equal efficacy in increasing bone mass compared to a control "no treatment" group that had no significant change in BMD.661
The common diuretics used post-transplant, thiazides and furosemide, have differing effects on bone and calcium metabolism. The thiazides have been described to counteract the adverse effect of steroids on calcium metabolism.662 However, furosemide—commonly used for control of hypertension and edema—causes calciuria and may potentially accelerate bone resorption. One study has demonstrated that thiazides decreased calcium loss in the urine and increased gut calcium absorption in patients receiving steroids.662 Other studies (in subjects not receiving steroids) have shown decreased bone resorption663 and a reduction in the prevalence of age-related hip fracture.664 Thiazides are associated with metabolic abnormalities (eg, hyperlipidemia, hypokalemia) also seen with steroids and, thus, they aggravate the steroid side-effects related to accelerated atherosclerosis. As a result, caution should be exercised with the use of thiazides in transplant recipients.
There is no FDA-approved therapy for post-transplant osteodystrophy. Therefore, recommendations for use of therapeutic agents approved for osteoporosis in the post-transplant situation is based on a small number of clinical trials in small numbers of patients and extrapolation from the nontransplant and kidney disease settings. The appropriate management of calcium and phosphate homeostasis in the post-transplant setting begins with a continuation of the principles and practices contained in the Guidelines for patients with chronic kidney disease and ESRD.
Much research is needed into all aspects of post-transplant osteodystrophy, including its pathogenesis and treatment. Most of the recommendations for management of post-transplant osteodystrophy require additional clinical trials. Clinical trials comparing alternative potentially beneficial therapies, alone or in combination, should be conducted in this important population.