Disturbances in mineral and bone metabolism are common in patients with CKD. A large body of evidence indicates that these derangements are associated with increased mortality and morbidity. These patients have bone pain, increased incidence of bone fractures and deformity, myopathy and muscle pain, and ruptures of tendons. Hyperphosphatemia also appears to be associated with increased mortality, and elevated blood levels of PTH exert significant adverse effects on the function of almost every organ.
Importantly, the long-term effects of these derangements on soft tissue calcification have become an area of growing concern in the care of CKD patients. Calcification of the lung leads to impaired pulmonary function, pulmonary fibrosis, pulmonary hypertension, right ventricular hypertrophy, and right-side congestive heart failure. Calcification of the myocardium, coronary arteries, and cardiac valves results in congestive heart failure, cardiac arrhythmias, ischemic heart disease, and death. Vascular calcification leads to ischemic lesions, soft-tissue necrosis, and difficulties for kidney transplantation.
The processes causing disordered mineral metabolism and bone disease have their onset in the early stages of CKD, continue throughout the course of progressive loss of kidney function, and may be influenced beneficially or adversely by the various therapeutic approaches now used. Thus, prevention of the disturbances in mineral and bone metabolism and their management early in the course of chronic kidney disease are extremely important in improving the quality of life and longevity of CKD patients.
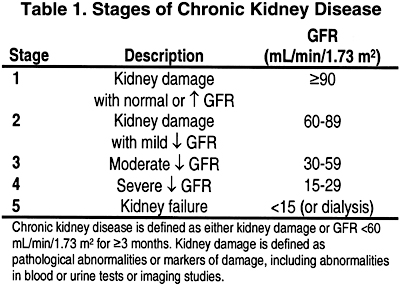
The present clinical practice guidelines were developed to provide an integrated clinical action plan to the management of this complex problem throughout the course of CKD. In the guidelines, the stages of CKD are defined according to the KDOQI Clinical Practice Guidelines for Chronic Kidney Disease: Evaluation, Classification, and Stratification.
The target population of these guidelines is adults (age 18 years and over) with CKD. While many of the recommendations made apply to all ages, there are sufficient issues unique to children and adolescents that a subcommittee of the Work Group of pediatricians has been constituted to address these issues. A separate set of these guidelines targeted to children and adolescents will be published separately.
The guidelines are based on a systematic review of the literature through January 1st, 2001. In formulating the guidelines, the rationale and evidentiary basis of each recommendation was made explicit. When all components of the rationale for a guideline were based on published evidence, the guidelines were labeled “Evidence.” When no definite evidence existed or the evidence was considered inconclusive, and either the guideline or steps in its rationale were based on judgment they were labeled “Opinion.” As such, it is the available literature that determined the labeling of each guideline. As a result, of the 111 recommendations made in these guidelines, about one third are evidence based and two thirds are opinion based. This distribution is true whether one considers the recommendations made for CKD stages 3 and 4 patients or for those in stage 5 who are already on maintenance dialysis. There are 8 recommendations in the guideline for the kidney transplant recipient; all of these 8 statements are opinion based.
Concerning opinion based statements, it is important to note that prior to their publication, a final draft of the guidelines was subjected to a broad-based review by experts, organizations, and the public. Thus, the chain of reasoning and recommendation of each opinion based guideline was exposed to open debate, with the final published product reflecting a wide consensus of healthcare professionals, providers, managers, organizations, associations, and patients.
No clinical practice guidelines, irrespective of the rigor of their development, can accomplish the intended improvement in outcomes without an implementation plan. Since the majority of the recommendations made in this set of guidelines are based on opinion, it is imperative that evaluation of their clinical outcomes be made a component of their implementation. In addition, the paucity of evidence based information in this field requires that a more integrated approach to research efforts be planned and conducted to provide answers to the many issues that remain to be elucidated. Actually, these are components of the implementation of these guidelines that has already been initiated. A coordinated approach to ongoing research and evaluation of the outcomes of the recommendations made should provide the answers necessary for the future updating of these guidelines.
Conversion Factors of Metric Units to SI Units
|
Test |
Metric Unit |
Conversion Factor |
SI Units |
| Calcuim | mg/dL | 0.25 |
mmol/L |
| Calcium ionized (serum) | mg/dL | 0.25 | mmol/L |
| Phosphorus (serum) | mg/dL | 0.32 | mmol/L |
| Magnesium (serum) | mg/dL | 0.41 | mmol/L |
| Creatinine (serum) | mg/dL | 83.30 | µmol/L |
| BUN (serum) | mg/dL | 0.36 | mmol/L |
| Albumin (serum) | g/dL | 10.00 | g/L |
| Alkaline phosphatase (serum) | IU/L | 0.02 | µkat/L |
| Intact parathyroid hormone (serum) | pg/mL | 0.11 | pmol/L |
| 25(OH)2D (serum or plasma) | ng/mL | 2.5 | nmol/L |
| 1.25(OH)2D (serum or plasma) | pg/mL | 2.4 | pmol/L |