CHRONIC KIDNEY disease is a worldwide public health problem. In the United States, there is a rising incidence and prevalence of kidney failure, with poor outcomes and high cost. There is an even higher prevalence of earlier stages of chronic kidney disease.
Increasing evidence, accrued in the past decades, indicates that the adverse outcomes of chronic kidney disease, such as kidney failure, cardiovascular disease, and premature death, can be prevented or delayed. Earlier stages of chronic kidney disease can be detected through laboratory testing. Treatment of earlier stages of chronic kidney disease is effective in slowing the progression toward kidney failure. Initiation of treatment for cardiovascular risk factors at earlier stages of chronic kidney disease should be effective in reducing cardiovascular disease events both before and after the onset of kidney failure.
Unfortunately, chronic kidney disease is “under- diagnosed” and “under-treated” in the United States, resulting in lost opportunities for prevention. One reason is the lack of agreement on a definition and classification of stages in the progression of chronic kidney disease. A clinically applicable classification would be based on laboratory evaluation of the severity of kidney disease, association of level of kidney function with complications, and stratification of risks for loss of kidney function and development of cardiovascular disease.
In 2000, the National Kidney Foundation (NKF) Kidney Disease Outcome Quality Initiative (KDOQI) Advisory Board approved development of clinical practice guidelines to define chronic kidney disease and to classify stages in the progression of chronic kidney disease. The Work Group charged with developing the guidelines consisted of experts in nephrology, pediatric nephrology, epidemiology, laboratory medicine, nutrition, social work, gerontology, and family medicine. An Evidence Review Team, consisting of nephrologists and methodologists, was responsible for assembling the evidence. The goals adopted by the Work Group are listed in Table 1.
Table 1. Goals of the CKD Work Group |
Definition of chronic kidney disease and Evaluation of laboratory measurements for the Association of the level of kidney function with Stratification of the risk for loss of kidney function |
Defining chronic kidney disease and classifying the stages of severity would provide a common language for communication among providers, patients and their families, investigators, and policy-makers and a framework for developing a public health approach to affect care and improve outcomes of chronic kidney disease. A uniform terminology would permit:
- More reliable estimates of the prevalence of earlier stages of disease and of the population at increased risk for development of chronic kidney disease
- Recommendations for laboratory testing to detect earlier stages and progression to later stages
- Associations of stages with clinical manifestations of disease
- Evaluation of factors associated with a high risk of progression from one stage to the next or of development of other adverse outcomes
- Evaluation of treatments to slow progression or prevent other adverse outcomes.
Clinical practice guidelines, clinical performance measures, and continuous quality improvement efforts could then be directed to stages of chronic kidney disease.
The Work Group did not specifically address evaluation and treatment for chronic kidney disease. However, this guideline contains brief reference to diagnosis and clinical interventions and can serve as a “road map,” linking other clinical practice guidelines and pointing out where other guidelines need to be developed. Eventually, KDOQI will include interventional guidelines. The first three of these, on bone disease, dyslipidemia, and blood pressure management are currently under development. Other guidelines on cardiovascular disease in dialysis patients and kidney biopsy will be initiated in the Winter of 2001.
This report contains a summary of background information available at the time the Work Group began its deliberations, the 15 guidelines and the accompanying rationale, suggestions for clinical performance measures, a clinical approach to chronic kidney disease using these guidelines, and appendices to describe methods for the review of evidence. The guidelines are based on a systematic review of the literature and the consensus of the Work Group. The guidelines have been reviewed by the KDOQI Advisory Board, a large number of professional organizations and societies, selected experts, and interested members of the public and have been approved by the Board of Directors of the NKF.
The Work Group defined “chronic kidney disease” to include conditions that affect the kidney, with the potential to cause either progressive loss of kidney function or complications resulting from decreased kidney function. Chronic kidney disease was thus defined as the presence of kidney damage or decreased level of kidney function for three months or more, irrespective of diagnosis.
The target population includes individuals with chronic kidney disease or at increased risk of developing chronic kidney disease. The majority of topics focus on adults (age ≥ 18 years). Many of the same principles apply to children as well. In particular, the classification of stages of disease and principles of diagnostic testing are similar. A subcommittee of the Work Group examined issues related to children and participated in development of the first six guidelines of the present document. However, there are sufficient differences between adults and children in the association of GFR with signs and symptoms of uremia and in stratification of risk for adverse outcomes that these latter issues are addressed only for adults. A separate set of guidelines for children will have to be developed by a later Work Group.
The target audience includes a wide range of individuals: those who have or are at increased risk of developing chronic kidney disease (the target population) and their families; health care professionals caring for the target population; manufacturers of instruments and diagnostic laboratories performing measurements of kidney function; agencies and institutions planning, providing or paying for the health care needs of the target population; and investigators studying chronic kidney disease.
There will be only brief reference to clinical interventions, sufficient to provide a basis for other clinical practice guidelines relevant to the evaluation and management of chronic kidney disease. Subsequent KDOQI clinical practice guidelines will be based on the framework developed here.
The Work Group developed he following operational definition of chronic kidney disease (Table 2).
Table 2. Definition of Chronic Kidney Disease |
Criteria |
|
| Abbreviation: GFR, glomerular filtration rate |
Table 3. Chronic Kidney Disease: A Clinical Action Plan |
|||
| Stage | Description | GFR (mL/min/1.73m2) |
Action* |
| At increased risk | ≥90 (with CKD risk factors) |
Screening CKD risk reduction |
|
| 1. | Kidney damage with normal or ↑ GFR |
≥90 |
Diagnosis and treatment Treatment of comorbid conditions, slowing progression, CVD risk reduction |
| 2. | Kidney damage with mild ↓ GFR |
60-89 | Estimating progression |
| 3. | Moderate ↓ GFR | 30-59 | Evaluating and treating complications |
| 4. | Severe ↓ GFR | 15-29 | Preparation for kidney replacement therapy |
| 5. | Kidney Failure | <15 (or dialysis) |
Replacement (if uremia present) |
Shaded area identifies patients who have chronic kidney disease; unshaded area designates individuals who are at increased risk for developing chronic kidney disease. Chronic kidney disease is defined as either kidney damage or GFR <60 mL/min/1.73 m2 for ≥ 3 months. Kidney damage is defined as pathologic abnormalities or markers of damage, including abnormalities in blood or urine tests or imaging studies. |
|||
Table 3 shows the classification of stages of chronic kidney disease, including the population at increased risk of developing chronic kidney disease, and actions to prevent the development of chronic kidney disease and to improve outcomes in each stage.
The word “kidney” is of Middle English origin and is immediately understood by patients, their families, providers, health care professionals, and the lay public of native English speakers. On the other hand, “renal” and “nephrology,” derived from Latin and Greek roots, respectively, commonly require interpretation and explanation. The Work Group and the NKF are committed to communicating in language that can be widely understood, hence the preferential use of “kidney” throughout these guidelines. The term “End-Stage Renal Disease” (ESRD) has been retained because of its administrative usage in the United States referring to patients treated by dialysis or transplantation, irrespective of their level of kidney function.
Currently, there is no uniform classification of the stages of chronic kidney disease. A review of textbooks and journal articles clearly demonstrates ambiguity and overlap in the meaning of current terms. The Work Group concluded that uniform definitions of terms and stages would improve communication between patients and providers, enhance public education, and promote dissemination of research results. In addition, it was believed that uniform definitions would enhance conduct of clinical research.
Adverse outcomes of kidney disease are based on the level of kidney function and risk of loss of function in the future. Chronic kidney disease tends to worsen over time. Therefore, the risk of adverse outcomes increases over time with disease severity. Many disciplines in medicine, including related specialties of hypertension, cardiovascular disease, diabetes, and transplantation, have adopted classification systems based on severity to guide clinical interventions, research, and professional and public education. Such a model is essential for any public health approach to disease.
Table 4. Stages and Prevalence of Chronic Kidney Disease (Age ≥ 20) |
||||
| Stage | Description | GFR (mL/min/1.73m2) |
Prevalence* | |
| At increased risk | ≥ 90 (with CKD risk factors) |
N(1000s) | % | |
| 1. | Kidney damage with normal or↑GFR |
≥ 90 | 5,900 | 3.3 |
| 2. | Kidney damage with mild ↓ GFR |
60-89 | 5,300 | 3.0 |
| 3. | Moderate ↓ GFR | 30-59 | 7,600 | 4.3 |
| 4. | Severe ↓ GFR | 15-29 | 400 | 0.2 |
| 5. | Kidney Failure | <15 (or dialysis) |
300 | 0.1 |
*Data for Stages 1-4 from NHANES III (1988-1994)1. Population of 177 million adults age ≥ 20 years. Data for Stage 5 from USRDS (1998)2 include approximately 230,000 patients treated by dialysis, and assume 70,000 additional pritents not on dialysis. GFR estimated from serum creatinine using MDRD Study equation based on age, gender, race and calibration for serum creatinine. For stages 1 and 2, kidney damage estimated by spot albumin-to-creatinine reatio >17 mg/g in men or >25 mg/g in women on two measurements. |
||||
The level of glomerular filtration rate (GFR) is widely accepted as the best overall measure of kidney function in health and disease. Providers and patients are familiar with the concept that “the kidney is like a filter.” GFR is the best measure of the kidneys’ ability to filter blood. In addition, expressing the level of kidney function on a continuous scale allows development of patient and public education programs that encourage individuals to “Know your number!”
The term “GFR” is not intuitively evident to anyone. Rather, it is a learned term, which allows the ultimate expression of the complex functions of the kidney in one single numerical expression. Conversely, numbers are an intuitive concept and easily understandable by everyone. It is fortunate then that once the term “GFR” is learned, the expression “Know your number!” becomes intuitive and easily understood.
Why Include an “Action Plan”?
Action is necessary to improve outcomes, which is the ultimate goal of the NKF. No clinical practice guideline, irrespective of the rigor of its development, can accomplish its intended improvement in outcome without an implementation plan. This has been the charge of the Advisory Board. The process has been set in motion in parallel with that of development of the guidelines.
Using the definition and stages of chronic kidney disease, the Work Group was able to provide rough estimates of the prevalence of each stage in adults from the Third National Health and Nutrition Examination Survey (NHANES III) (Table 4). Methods for estimating prevalence are detailed in Part 10, Appendix 2. The prevalence of chronic kidney disease in children is too low to provide accurate estimates of prevalence of each stage based on data from NHANES III.
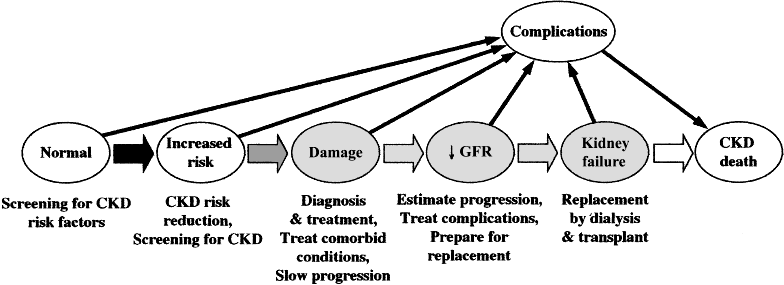
Fig 1.
Fig 1. Evidence model for stages in the initiation and progression of chronic kidney disease, and therapeutic interventions. Shaded ellipses represent stages of chronic kidney disease; unshaded ellipses represent potential antecedents or consequences of CKD. Thick arrows between ellipses represent factors associated with initiation and progression of disease that can be affected or detected by interventions: susceptibility factors (black); initiation factors (dark gray); progression factors (light gray); and end-stage factors (white). Interventions for each stage are given beneath the stage. Individuals who appear normal should be screened for CKD risk factors. Individuals known to be at increased risk for CKD should be screened for CKD. Modified and reprinted with permission3
The framework that has been adopted can be used to develop an evidence model of the course of chronic kidney disease (Fig 1). It is anticipated that clinical practice guidelines for interventions to reduce adverse outcomes in patients with chronic kidney disease can be based on this model.
This line of logic allows for the ultimate construction of a list of modifiable risk factors at each stage of chronic kidney disease, as shown in Table 5.
The guidelines developed by the Work Group are based on a systematic review of the literature using an approach based on the procedure outlined by the Agency for Healthcare Research and Quality (formerly the Agency for Health Care Policy and Research) with modifications appropriate to the goals. An Evidence Review Team was appointed by the NKF to collaborate with the Work Group to conduct a systematic review of the literature on which to base the guidelines. A detailed explanation of these methods is provided in Part 10, Appendices 1 and 2; Table 6 provides a brief listing of the steps involved in this approach.
Table 6. Approach to the Evidence Review |
Develop and refine topics; |
Determine approach to topics:
New concepts - systematic review of original articles and analysis of primary data, if available. |
Retrieval of evidence (literature review); |
Analysis of primary data from the Third National Health and Nutrition Examination Survey (NHANES III) and other sources; |
Evaluation of evidence (types and quality); |
Synthesis of evidence (tables); |
Translation of evidence into clinical practice guidelines; |
Identification of guidelines suitable for translation into clinical performance measures; |
Public review and revisions; |
Approval by Board of Directors of the NKF |
A uniform format for summarizing the strength of evidence has been developed using four dimensions: study size, applicability, results, and methodological quality.
An example of an evidence table is shown in the above table. Within each table, studies are ordered first by methodological quality (best to worst), then by applicability (most to least), and then by study size (largest to smallest). Detailed evidence tables are on file at the National Kidney Foundation.
Applicability
Applicability (also known as generalizability or external validity) addresses the issue of whether the study population is sufficiently broad so that the results can be generalized to the population of interest at large. The study population is typically defined by the inclusion and exclusion criteria. The target population was defined to include patients with chronic kidney disease and those at increased risk of chronic kidney disease, except where noted. A designation for applicability was assigned to each article, according to a three-level scale. In making this assessment, sociodemographic characteristics were considered, as were the stated causes of chronic kidney disease and prior treatments.
GFR Range
For all studies, the range of GFR (or creatinine clearance [CCr]) is represented graphically when available (see table above). The mean or median GFR is represented by a vertical line, with a horizontal bar showing a range that includes approximately 95% of study participants. Studies without a vertical or horizontal line did not provide data on the mean/ median or range, respectively. When GFR or CCr measurements are not available, serum creatinine levels are listed as text.
Results
Results are represented by prevalence levels, proportions (percents) for categorical variables, mean levels for continuous variables, and associations between study measures. Symbols indicate the type and significance of associations between study measures:
The specific meanings of these symbols are explained in the footnotes of tables where they appear. Some informative studies reported only single point estimates of study measures (eg, mean data) rather than associations. Where data on associations were limited, evidence tables provide these point estimates. Studies that provide data on associations and studies that provide only point estimates are listed and ranked separately, with shading used to distinguish them (as in the table, Example of Format for Evidence Tables).
Quality
Methodological quality (or internal validity) refers to the design, conduct, and reporting of the clinical study. Because studies with a variety of types of design were evaluated, a three level classification of study quality was devised:
Strength of Evidence
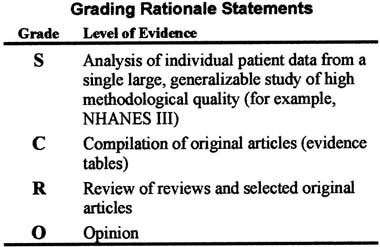
Each rationale statement has been graded according the level of evidence on which it is based.
Guideline statements are grouped into four parts, corresponding to the four goals of the CKD Work Group. Guideline statements are reproduced in the Executive Summary. The reader is referred to specific pages for rationale, evidence tables and references.
Chronic kidney disease is a major public health problem. Improving outcomes for people with chronic kidney disease requires a coordinated world-wide approach to prevention of adverse outcomes through defining the disease and its outcomes, estimating disease prevalence, identifying earlier stages of disease and antecedent risk factors, and detection and treatment for populations at increased risk for adverse outcomes. The goal of Part 4 is to create an operational definition and classification of stages of chronic kidney disease and provide estimates of disease prevalence by stage, to develop a broad overview of a “clinical action plan” for evaluation and management of each stage of chronic kidney disease, and to define individuals at increased risk for developing chronic kidney disease. Studies of disease prevalence were evaluated as described in Appendix 1, Table 147. Data from NHANES III were used to develop estimates of disease prevalence in adults as described in Appendix 2.