The assessment and treatment of both risk factors and end organs are essential in the management of cardiovascular diseases. The first section will deal with the end organs and will focus on cardiac, cerebrovascular and peripheral vascular diseases. Cardiac diseases have justifiably received the most attention because they are by far the most common cause of cardiovascular deaths in dialysis patients. Cerebrovascular diseases and peripheral vascular diseases, however, also lead to substantial morbidity and mortality and have often been overlooked by practitioners and clinical researchers.
The workgroup has faced dilemma in the scope and depth of the coverage of end organ diseases. There has been only one small randomized trial that demonstrated beneficial effects of specific cardioprotective drugs (namely, carvedilol) published in dialysis patients. Therefore, most guidelines described in this section are referred from published guidelines in the general population. Nonetheless, there are unusual features in the dialysis patients that the practitioners need to be aware of. For example, the pathophysiology and rate of progression of cardiac valvular calcification appear to be different from those in the general population. Surveillance and treatment strategies should take these caveats into consideration. On the other hand, the implant of tissue valves is proscribed in the existing ACC/AHA guidelines. More recent and stronger evidence, however, suggest that tissue valves are associated with equivalent outcomes in dialysis patients. These similarities, not only differences, between dialysis patients and the general population also need to be emphasized.
The section on end organ diseases is written for not only the nephrologists, but also the general practitioners, cardiologists, vascular surgeons and other practitioners.
Guideline 4: Chronic Coronary Artery Disease
The processes by which atherosclerotic disease may be exacerbated by the uremic milieu, and the outcomes of patients on dialysis with established CAD, are worse than outcomes in the general population.
4.1 The medical management of chronic CAD in dialysis patients should follow that of the general population. In particular, patients should receive acetylsalicylic acid (ASA), beta-blockers, nitroglycerin, ACE inhibitors or angiotensin receptor blockers (ARB), statins, and/or calcium-channel blockers (CCB) as indicated. Dose adjustments are required for medications that are renally excreted or dialyzed. (C)
4.2 Unique aspects of management in the dialysis population include:
4.2a Maintenance of hemodynamic dry weight. (C)
4.2b Maintenance of hemoglobin levels in accordance with KDOQI Guidelines.52(B)
4.2c Modification of dosing regimens so that cardiovascular medications do not adversely impact the delivery of dialysis and ultrafiltration. Nocturnal dosing of medications should be considered. (C)
4.2d Loop diuretics to increase urine output may be helpful for those patients with substantial residual renal function. (C)
4.3 In patients with obstructive CAD lesions, PCI and CABG are appropriate revascularization techniques. (C)
4.3a Drug-eluting or conventional stents should be implemented according to local practice. The incidence of restenosis after PCI with drug-eluting stents is reduced in the nondialysis population. As the risk of restenosis is higher in dialysis patients, the use of drug-eluting stents is favored.
4.3b Patients with three-vessel and/or Left main disease should undergo CABG as preferred therapy. (C)
Management of CAD (Weak)
Maintenance of target dry weight is important for the management of heart disease. Target dry weight should be periodically assessed because it may change over time. This is particularly true for diabetic and elderly patients, since their muscle mass may decline over time. Caution should be exercised when using nitrates in low preload states (e.g., hypovolemia at the end of HD session), as these states may potentiate the hypotensive effect of the drug. The hemodynamic and electrophysiological effects of CCBs are markedly different from each other, and these differences should be evaluated when selecting a suitable therapy.
Clopidogrel is approved in the general population for the secondary prevention of atherosclerotic cardiovascular disease (ASCVD) events, including CAD. Most dialysis patients would theoretically be candidates for long-term clopidogrel therapy. It should be prescribed for all patients with coronary stents and considered in other patients with stable CAD or established ASCVD. All dialysis patients with CAD who are not allergic to ASA should receive ASA. The efficacy-to-risk ratio of ASA in combination with clopidogrel—compared to ASA alone—for the secondary prevention of ASCVD events is unknown in dialysis patients; one undefined risk is hemorrhage. There are data indicating a two-fold relative hemorrhagic risk with ASA+clopidogrel versus placebo alone.66 Since it may significantly increase the risk of hemorrhage, clopidogrel should be withheld (typically for 1 week) before major elective surgery. In contrast, withholding ASA before surgery is usually unnecessary. Since the use of clopidogrel is mandatory for at least 30 days after coronary stent placement, elective major surgery—including renal transplantation—should generally be postponed to allow for discontinuation of clopidogrel before surgery. For this reason, in the immediate poststent period (when clopidogrel is required), it may be appropriate to temporarily suspend the active waitlist status of patients awaiting cadaveric renal transplantation until the clopidogrel can be discontinued. This decision should be made by consultation of the patient’s nephrologist, cardiologist, and transplant surgeon (with attention to the clinical profile of the particular patient).
Coronary revascularization (Weak)
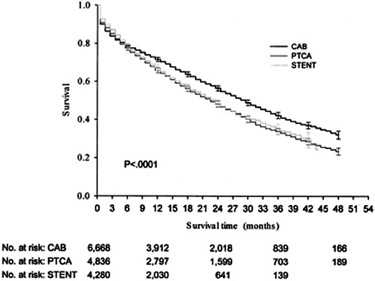
The short-term and long-term mortality after coronary revascularization procedures in dialysis patients is considerably higher than those in the general population. Coronary revascularization can be performed with either surgical or percutaneous approaches. In diabetic dialysis patients, there is no difference in survival between percutaneous angioplasty with and without stenting. In contrast, stents offer better outcomes than angioplasty without stents in nondiabetic dialysis patients. In either diabetic or nondiabetic patients, the mortality at 6–9 months in retrospective studies is higher after PCI, compared to coronary bypass surgery, although the mortality after PCI is lower within 90 days after the procedure (see Fig 2 and Table 1).55,67 Observational studies support the conclusion that surgical coronary revascularization is associated with better outcomes than percutaneous coronary intervention in dialysis patients.67–69 The survival advantage of surgical coronary bypass over PCI in dialysis patients is attributable to the use of internal mammary artery bypass grafts.70 Therefore, dialysis patients most likely to benefit from coronary bypass surgery are those who are suitable candidates for internal mammary graft utilization. In dialysis patients not receiving internal mammary grafts, there is no apparent survival advantage compared to PCI, but there is still a reduced rate of repeat coronary revascularization. There are currently no data on the impact of coronary brachytherapy or drug-eluting stents on re-stenosis after PCI in dialysis patients, but these techniques may improve the long-term outcome of dialysis patients after PCI.
Figure 2 - Estimated All-Cause Survival of Dialysis Patients after CABG, PTCA, and Stenting
These general trends notwithstanding, the selection of coronary artery revascularization techniques should also be guided by local institutional experience, since a wide variety of outcome data from single centers has been reported in the literature.
The risk of re-stenosis after percutaneous coronary angioplasty and stent placement is higher in dialysis patients than in the general population (see Table 1).55,71 The failure rate of various types of coronary grafts has not been studied in angiographic series in dialysis patients. In addition, re-stenosis in dialysis patients may not be clinically apparent, since dyspnea and angina can occur in the setting of volume overload. Therefore, in all dialysis patients who have undergone PCI, provocative stress imaging should be considered to detect clinically silent re-stenosis 12–16 weeks after PCI. This latter recommendation may be modified as more data on drug-eluting stents in dialysis patients become available.