The following sections have been prepared to ensure that the state of the art and science related to CVD includes novel concepts, therapeutic strategies, and emerging areas of pathophysiological and practical importance to the care of dialysis patients.
The reader will notice that the format of this section is different, reflecting its different perspective: namely, the relative lack of evidence on which to base plausible guideline statements. The evidence that does exist, and is cited in this section, is either completely in nondialysis populations, or is purely associative information, with no intervention in any population yet tested. Thus, it would be a problem to include guideline statements or recommendations.
Nonetheless, this section describes the current status of knowledge with respect to risk factors and biomarkers, and represents an overview of key areas for future clinical trials. The reader is encouraged to review this section, and examine his or her current understanding and practice within the context of these highlights.
The literature review has been conducted using the same systematic strategy as for the previous guidelines in this document. The reviews presented here have been thoughtfully constructed so that clinicians can adopt different practices based on them. However, for reasons cited above, the ability to truly recommend or suggest changes in practice would be premature at this time.
Intradialytic hypotension (IDH) is defined as a decrease in systolic blood pressure by ≥20 mm Hg or a decrease in MAP by 10 mm Hg associated with symptoms that include: abdominal discomfort; yawning; sighing; nausea; vomiting; muscle cramps; restlessness; dizziness or fainting; and anxiety. It impairs the patient’s well-being, can induce cardiac arrhythmias, predisposes to coronary and/or cerebral ischemic events. In addition, IDH precludes the delivery of an adequate dose of dialysis, as hypotension episodes lead to the compartment effect and result in suboptimal Kt/Vurea.
Cardiovascular complications of IDH include: ischemic (cardiac or neurological) events; vascular access thrombosis; dysrhythmias; and mesenteric venous infarction.364 Long-term effects of IDH include: volume overload due to suboptimal ultrafiltration and use of fluid boluses for resuscitation; LVH, with its associated morbidity and mortality; and interdialytic hypertension.
Evaluation of risk
During the past 10 years, despite improvements in dialysis technology, the frequency of IDH has remained unchanged at about 25% of all HD sessions.365 In addition, the incidence of IDH will continue to increase as an increasing number of elderly patients will develop CKD, and also due to the progressive increase in the number of diabetic patients with CKD. Patient subgroups most likely to have IDH include those with diabetic CKD, CVD, poor nutritional status and hypoalbuminemia, uremic neuropathy or autonomic dysfunction, severe anemia, age ≥65, and predialysis systolic blood pressure <100 mm Hg.
There are no large-scale, epidemiological studies to define the risk factors that are associated with the risk of developing IDH, although IDH appears to be more common in patients with diabetes and predialysis hypotension. Both normotensive or hypertensive dialysis patients can develop IDH. The degree of IDH in the same patient may vary from time to time or may have seasonal variations.
A small group of patients (5%-10%) may have low systolic blood pressure (<100 mm Hg) at the initiation of dialysis.364 This group includes anephric patients, those who are on dialysis for a longer period, and diabetic patients with persistent orthostatic hypotension due to autonomic dysfunction. Patients on dialysis with autonomic dysfunction show an exaggerated drop in systolic and diastolic blood pressures and MAP, compared to those without underlying autonomic dysfunction.366 Other risk factors include older age (>60 years), female sex, diabetes mellitus, presence of CAD, and the use of nitrates before a dialysis session.
Patients with CKD have defective reactivity of the resistance vessels as well as the capacitance vessels during the HD sessions.367,368 The exact mechanism of this poor vascular responsiveness is not known; however, recent data from isolated ultrafiltration and hemodiafiltration have shown that vascular responses remained intact as these modalities are not associated with increase in core body temperature.369
The following subgroups of chronic HD patients should be evaluated carefully for the risk of developing IDH:
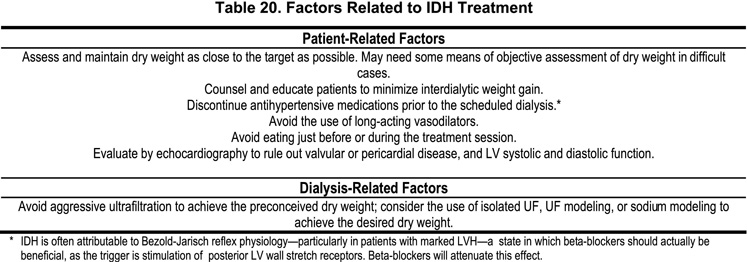
Routine measures for the treatment of IDH include the use of Trendenlenburg’s position and saline boluses to increase the systolic blood pressure to 100–110 mm Hg. In addition, it is advisable to assess for the signs of orthostatic hypotension before the patient is discharged from the dialysis unit. Additional factors relating to IDH treatment are presented in Table 20.
Dialysate temperature modeling
During standard dialysis, an increase in core body temperature is usual and increases the risk for IDH. The increase in body temperature is either related to heat load from the extracorporeal system, or secondary to volume removal. Volume removal is associated with increased metabolic rate with decreased thermal losses either directly, or secondary to peripheral vasoconstriction370 and impaired convective mechanisms of heat loss.
Temperature monitoring is difficult in dialysis patients due to variability in room, core body, and dialysate temperatures, as well as the lack of sensitive equipment to monitor the dialysate-blood temperature gradient. The use of low-temperature dialysate (i.e., lower than the patient’s core temperature) compared with standard dialysate-temperature (37–38°C)371,372 decreases the frequency and intensity of symptomatic hypotension. Low-temperature dialysis improves the reactivity of capacitance and resistance vessels, and is associated with improvement in cardiac contractility.373,374
Isothermic dialysis
Maintenance of isothermic dialysis involves keeping the temperature constant during the dialysis treatment. Each percent change in ultrafiltration-induced body weight requires removal of 6% heat to prevent an increase in core body temperature.370 It was shown that differences in vascular reactivity in patients with standard HD, hemodiafiltration, or isolated ultrafiltration remained unchanged if energy transfer was similar.369,375
In a recent multicenter analysis, the impact of thermoneutral dialysis (preventing any transfer of thermal energy between dialysate and extracorporeal circulation) on hemodynamic stability in selected hypotension-prone patients was compared to isothermic dialysis (keeping the predialysis body temperature unchanged) by using blood-temperature monitoring. The frequency of intradialytic morbid events decreased by 25% with isothermic HD.376 During isothermic treatments, body temperature was maintained at predialysis temperature settings and was tolerated without adverse effects, as compared to a simple decrease in dialysate temperature that often leads to rigors and shivering.377
Dialysate calcium modeling
The long-term hemodynamic and osseous consequences associated with the use of different levels of dialysate calcium need careful evaluation. The use of low-calcium dialysate has been associated with decreased LV contractility and a corresponding decrease in blood pressure.378,379 It was further associated with a significant intradialytic decrease in blood pressure in both healthy and cardiac-compromised HD patients and patients with decreased LV ejection fraction.378,380 Significant changes in blood pressure,380 myocardial contractility378,380,381 and changes in intradialytic blood pressure in cardiac compromised patients382 have been associated with the changes when dialysate calcium concentration is ≤2.5 mEq/L. A dialysate Ca of 3.5 mEq/L may lead to hypercalcemia and decreased bone turnover.78 Furthermore, limited studies have shown only marginal benefit on the frequency of IDH episodes with the use of dialysate Ca >3.0 mEq/L.
Dialysate sodium modeling
Sodium profiling is based on the principle that there is a linear relationship between the changes in plasma sodium concentration and blood volume (BV). The intradialytic decrease in plasma volume can be as much as three-fold greater with dialysate sodium of 134 mEq/L than with a dialysate sodium of 144 mEq/L.383–386 In this technique, the dialysate sodium concentration at the beginning of treatment is hypertonic, and during the final hours of dialysis is progressively reduced, reaching almost normal levels before the end of dialysis.
Sodium modeling prevents the development of IDH by: a) an increased ECF sodium level at the time of peak UF rate improves shift of water from ICF to the ECF compartment with improved venous refill and prevention of the Bezold-Jarisch reflex;387,388 and b) hypertonic dialysate—to a greater extent—accelerates urea equilibration between ICF and ECF while urea removal is at its peak during the first hour of dialysis.389
Limitations of dialysate sodium modeling include the following: a) there is poor temporal correlation between the time of onset of IDH and an antecedent decrease in blood volume;390 b) there is a significant interdialysis and interindividual variation in serum sodium, and for any given level of serum sodium, the amount of diffusible plasma water varies based on total body water, serum proteins and other nondiffusible elements in the plasma;391 and c) the development of postdialysis hypernatremia can be associated with thirst, dysphoria, hypertension, and increased interdialytic weight gain.384 In some instances, a reverse sodium profile is prescribed, in which the dialysate sodium concentration increases toward the end of the session when plasma volume is lowest. Various profiles of ultrafiltration modeling can also be used to decrease the incidence of hypernatremia.
Midodrine
Midodrine prevents IDH by maintaining the central blood volume (CBV) and cardiac output, and a marginal increase in peripheral vascular resistance (PVR). A single dose of midodrine (5 mg) administered 30 minutes before the dialysis session was associated with an improvement in intradialytic and postdialytic systolic and diastolic blood pressures and MAP, compared to dialysis sessions without the use of Midodrine.392 Others have reported the continued efficacy of midodrine use for more than 8 months without development of adverse events.393
Midodrine is effectively cleared by HD and its half-life is reduced to 1.4 hours by HD.394 Such pharmacokinetic data are not available in PD patients at the time of writing these guidelines. Midodrine has minimal cardiac and central nervous system effects, due to its specificity for α1 receptors, and it does not cross the blood-brain barrier. The most frequent side effects of midodrine are piloerection, scalp itching or tingling, nausea and heartburn, urinary urgency, headache, nervousness, and sleep disturbance. Long-term use has been associated with supine systolic hypertension in less than 10% of patients; this side effect warrants cessation of therapy.395 Patients should also be monitored for bradycardia, as midodrine is associated with reflex parasympathetic stimulation.396 Since midodrine is administered on the days of dialysis, both prodrug and active metabolite are removed effectively by HD; therefore, the risk of developing supine hypertension is possible, but very rare.
Midodrine should be used cautiously in patients with CHF and in those using other negative chronotropic agents such as beta-blockers, digoxin and nondihdropyridine CCBs. Concomitant use with other α-adrenergic agents—such as ephedrine, pseudoephedrine and phenylpropanolamine—should be avoided, as this may aggravate supine hypertension. Midodrine can also antagonize the actions of α-adrenergic blockers (such as terazosin, prazosin and doxazosine) and could result in urinary retention.
The combination of cool dialysate and predialysis doses of midodrine may lead to decreased frequency and intensity of symptoms of IDH without side effects.
Carnitine
Hemodialysis therapy for more than 6 months is associated with reduction of plasma and tissue levels of carnitine and carnitine esters. Carnitine deficiency is associated with several metabolic defects, defined as dialysis-related carnitine disorders, including IDH.397 A multicenter trial of intravenous L-carnitine therapy at 20 mg/kg into the dialysis venous port with each session of dialysis was associated with reduced frequency of IDH and muscle cramps (44% versus 18% and 36% versus 13%, respectively), as compared with the placebo group.397–399 The reasons for this beneficial effect are not clear, but could be due to improvement in vascular smooth muscle and cardiac muscle function.
Sertraline
Sertraline is a selective serotonin reuptake inhibitor and has been shown to improve symptoms in patients with neurocardiogenic syncope,400 idiopathic orthostatic hypotension,401 and IDH.402 These disorders share a common pathogenic mechanism with IDH: a paradoxical withdrawal of central sympathetic outflow, resulting in sudden decrease in blood pressure with bradycardia. Both retrospective and prospective studies in small number of patients demonstrated that treatment with sertraline hydrochloride was associated with an improvement in the hemodynamic parameters in patients with IDH.402–404 Side effects of sertraline include dizziness, insomnia, fatigue, somnolence, and headache.
Resistant IDH
Resistant cases of IDH should be treated with a combination of modalities, such as combination of midodrine and dialysate temperature profiling, combination of dialysate temperature profiling and 3 mEq/L dialysate calcium, or combination of dialysate temperature modeling and sodium modeling. Such patients should also be offered alternative measures to prevent and treat IDH. For example, isolated ultrafiltration and other techniques providing a high convective solute transport (such as hemofiltration and hemodiafiltration) are associated with decreased incidence of IDH and improved hemodynamic stability compared to conventional HD, due to improved plasma refill and appropriate neurohormonal response to loss of intravascular volume.236,405–407
There are limited data to make any recommendation about the benefit of extended daily dialysis or nocturnal HD to prevent the development of IDH. However, these two modalities of dialysis therapy have the advantage of slow ultrafiltration rate and the possibility to prevent the activation of Bezold-Jarisch reflex and subsequent cardiodepressor response. However, more clinical studies are needed to prove the efficacy and cost-effectiveness of these two modalities in the treatment of IDH.408–410
It is unclear if episodes of IDH, per se, are associated with increased morbidity and mortality. The data supporting the effectiveness of various therapeutic options for the treatment of IDH are available in the form of case series and case reports. Very few multicenter randomized studies have been published.
Objective assessment of dry weight using such methods as IVC sonography, or bioimpedance or tissue impedance techniques, have not been rigorously tested in relation to IDH and long-term clinical outcomes.
A randomized study in patients with IDH is needed to assess the safety, efficacy, and cost-effectiveness of automated feedback systems that continuously adjust ultrafiltration rate, dialysate sodium, and dialysate temperature. Controlled studies are also needed to examine the use of continuous on-line hematocrit monitoring to calculate the rate of ultrafiltration and blood volume and impedance measurements in the assessment of actual dry weight and desired goal for ultrafiltration.
Patients with CKD who are at risk for IDH may require evaluation for the presence of underlying cardiovascular and autonomic function. The patients’ medications list should be verified very carefully to avoid the use of short-acting anti-hypertensive medications and peripheral vasodilators immediately before the dialysis session.
Hemodialysis patients at risk for, or predisposed to, IDH may benefit from lowering dialysate temperature, dialysate sodium modeling, and maintaining dialysate calcium at 3 mEq/L. Further benefits may be derived from treatment with pharmacological agents that prevent the development of IDH.
If modifications in dialysis prescription and adjustments in antihypertensive medications do not improve IDH, these patients should be considered for extended daily dialysis or nocturnal HD. If no improvement is seen after these measures, patients may be counseled for living-donor kidney transplantation.