The following sections have been prepared to ensure that the state of the art and science related to CVD includes novel concepts, therapeutic strategies, and emerging areas of pathophysiological and practical importance to the care of dialysis patients.
The reader will notice that the format of this section is different, reflecting its different perspective: namely, the relative lack of evidence on which to base plausible guideline statements. The evidence that does exist, and is cited in this section, is either completely in nondialysis populations, or is purely associative information, with no intervention in any population yet tested. Thus, it would be a problem to include guideline statements or recommendations.
Nonetheless, this section describes the current status of knowledge with respect to risk factors and biomarkers, and represents an overview of key areas for future clinical trials. The reader is encouraged to review this section, and examine his or her current understanding and practice within the context of these highlights.
The literature review has been conducted using the same systematic strategy as for the previous guidelines in this document. The reviews presented here have been thoughtfully constructed so that clinicians can adopt different practices based on them. However, for reasons cited above, the ability to truly recommend or suggest changes in practice would be premature at this time.
Lipoprotein(a) [Lp(a)] is an LDL-like lipoprotein, consisting of an LDL particle to which the glycoprotein apolipoprotein(a) [apo(a)] is attached. Apolipoprotein(a) shows a high homology with plasminogen and competes with it for binding on plasminogen receptors, fibrinogen, and fibrin.584 This apolipoprotein contains a heritable number of so-called kringle-IV (K-IV) repeats, providing the basis for the apo(a) K-IV repeat polymorphism.585 The molecular weight of apo(a) increases with the number of K-IV repeats (300 kDa to >800 kDa) and is inversely related to the Lp(a) plasma concentrations. That means that individuals with high molecular-weight (HMW) or large apo(a) isoforms have, on average, low Lp(a) concentrations, and those with low molecular-weight (LMW) or small isoforms exhibit usually high concentrations of Lp(a). Depending on the population under investigation, this association explains between 30%- 70% of the variability in Lp(a) levels.
Since most studies showed that lipids were not useful for atherosclerosis risk assessment in dialysis patients, many studies during the last decade focused on nontraditional lipid abnormalities. Lp(a) was a promising candidate because of the strong evidence from the general population that Lp(a) is a risk factor for CVD.586–589
The NKF-KDOQI Clinical Practice Guidelines for Managing Dyslipidemias in CKD Patients51 focused primarily on lipids, and less on those abnormalities that cannot be interventionally influenced at present. Due to the strong interest in Lp(a), this review examines the literature relating Lp(a) and/or the apo(a) polymorphism to CVD.
Lp(a) concentrations and apo(a) size polymorphism in renal disease
In the early stages of renal disease, Lp(a) starts to increase, often long before glomerular filtration rate is decreased.590,591 This holds true mostly for patients with HMW apo(a) isoforms and not for those with LMW apo(a) isoforms when compared to apo(a) isoform-matched controls.322,590,592–595 This isoform-specific increase was observed in several-but not all-studies in non-nephrotic renal disease and HD patients, but not in patients with nephrotic syndrome596,597 or in PD patients.594 Those treatment groups showed an increase in Lp(a) in all apo(a) isoform groups, probably as a consequence of the pronounced protein loss they experience. In support of this assumption, a decrease of Lp(a) following a successful kidney transplantation can be observed in HD patients with HMW apo(a) isoforms598,599 and in CAPD patients with all apo(a) isoform groups.600
There is evidence that malnutrition and/or inflammation have an Lp(a)-increasing effect.322,434,601,602 However, the elevation of Lp(a) can be observed already in the earliest stages of renal impairment590 as well as in HD patients322 with HMW apo(a) phenotypes and normal CRP and/or normal serum amyloid A levels. These results suggest that CRP only modifies Lp(a) concentrations, but they fail to explain the apo(a) phenotype-specific elevation of Lp(a).
Association of Lp(a) concentrations with CVD
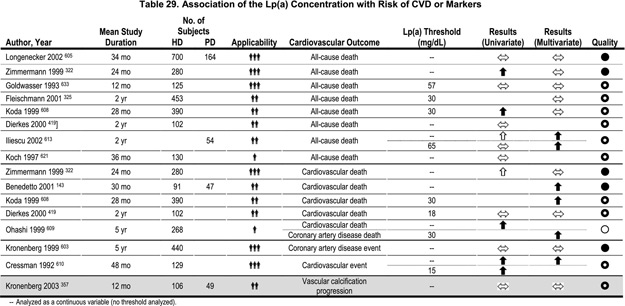
The association of Lp(a) with atherosclerotic complications was investigated in numerous studies in dialysis patients. The results, however, were ambiguous in prospective as well as in retrospective studies.143,159,322,325,357,419,555,561,603–633 Most of the retrospective studies, including those with the largest patient numbers, found no association between Lp(a) levels and cardiovascular complications. The same holds true for prospective studies (Table 29). A study of 129 HD patients reported significantly higher Lp(a) concentrations in those who suffered a CVD complication during the 4-year observation period.610 The two largest prospective studies, however, did not observe an association between high Lp(a) concentrations and CAD events603 or total mortality,605 respectively.
Association of the apo(a) size polymorphism with CVD
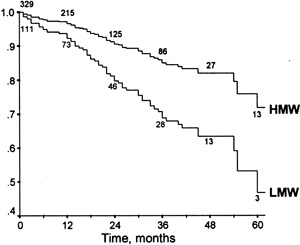
Almost all studies that did not only measure Lp(a) concentrations but also performed apo(a) phenotyping consistently showed an association between the apo(a) K-IV repeat polymorphism and CVD complications (Table 30).357,603–605,613,617–619,624,630 A study of 167 HD patients reported the apo(a) phenotype to be a better predictor for the prevalence and the degree of carotid atherosclerosis than the Lp(a) plasma concentration.618 Similarly, others found LMW apo(a) isoforms (besides age and oxidized LDL) to be predictive for the presence of carotid plaques in 109 predialysis patients with terminal chronic renal failure.434 A doubling of the frequency of LMW apo(a) phenotypes was observed in those CAPD patients who had suffered a CAD event.630 A large cross-sectional study in 607 HD patients described an association of LMW apo(a) phenotypes with CAD events.604 Another study of 440 HD patients prospectively followed for a period of 5 years found a strong association between the LMW apo(a) phenotype and CAD events defined by stringent criteria (definite myocardial infarction, percutaneous transluminal coronary angioplasty, aortocoronary bypass or a stenosis >50% in the coronary angiography) (Fig 7).603 Patients with LMW apo(a) isoforms had, on average, twice the number of coronary events per 100 patient-years.603 Similarly, the CHOICE study recently reported that LMW apo(a) isoforms were associated with total mortality in an inception cohort of 864 incident dialysis patients who were followed for a median of 33.7 months; again, Lp(a) concentrations were not associated with total mortality.605 On the other hand, when prevalent atherosclerotic CVD at the start of renal replacement therapy was investigated in the same cohort, Lp(a) concentrations were associated with prevalent disease in whites younger than 60 years, but not among blacks or those older than 60 years. In addition, apo(a) isoforms were not associated with prevalent atherosclerotic CVD.606
Figure 7 - Coronary Event-Free Survival and Apo(a) Phenotypes. Adjusted results are obtained from a multiple Cox proportional hazards regression analysis. Numbers near the survival curves represent the number of patients with HMW and LMW apo(a) phenotypes at risk at the times 0, 12, 24, 36, 48 and 60 months. Reproduced with permission. 603
Considering all these studies, it seems that Lp(a) concentrations might not be very fruitful for risk prediction. However, from two prospective studies (total of 1,300 patients) and most of the cross-sectional studies (including more than 1,000 patients) the evidence is strong that the apo(a) size polymorphism is associated with various endpoints. The diverging results for Lp(a) concentrations might be caused, at least in part, by the methodological problems with the Lp(a) measurement, which is not standardized. The apo(a) polymorphism might be a better predictor as discussed previously.603,634 This fact is based on the above-described apo(a) isoform-specific elevation of Lp(a).592,594 In hemodialysis patients with only large apo(a) isoforms, Lp(a) concentrations increase and come closer to the concentrations usually seen in patients with small isoforms. Therefore, the risk for atherosclerotic complications can no longer be discriminated by means of Lp(a) concentrations. The apo(a) phenotype, however, gives approximate information about the prior contribution of Lp(a) to the risk for atherosclerosis. This is probably more important, since the predisease period (with its specific atherosclerosis risk) lasted longer in most of the patients than did the present situation. It is furthermore conceivable that patients with a LMW apo(a) phenotype and a more pronounced atherosclerosis preload develop a more rapidly-progressing atherosclerosis after commencement of renal insufficiency or hemodialysis treatment.
At present, no easily practicable method for lowering Lp(a) is available and sufficient proof is lacking that lowering Lp(a) is favorable. This holds true for the general population, as well as dialysis patients. The question remains whether the apo(a) K-IV repeat polymorphism should be determined in dialysis patients.
Further large dialysis cohorts should investigate the value of Lp(a) concentrations and Apo(a) phenotypes for risk assessment. This question should especially be addressed in PD patients as well as in various ethnicities. A possible interaction of various apo(a) isoforms with lipids and other cardiovascular risk factors should be investigated. Experimental therapeutic strategies to lower Lp(a) should be examined in randomized, controlled clinical trials, especially in high-risk populations such as dialysis patients.
Although Lp(a) levels are not a suitable factor for CVD risk prediction in dialysis patients, there is strong evidence that the apo(a) size polymorphism is associated with various clinical endpoints.