
Serum GH levels are elevated in uremia, yet growth retardation is a frequent accompaniment of chronic renal insufficiency (CRI) in infants, children, and adolescents. An apparent GH-resistant state is thought to result from a combination of reduced GH-receptor expression, especially in the liver, with subsequent decreased IGF-I production and increased IGF-binding protein levels, which reduce the availability of free IGF-I. Because IGF-I is the primary stimulus for the increase in linear growth, it is probable that both reduced hepatic GH-receptor expression and increased IGF-binding protein levels contribute to the GH-resistant state in uremia.66,67
Pharmacologic doses of exogenous recombinant hGH (0.05 mg/kg/d; Genentech, South San Francisco, CA; 30 IU/m2/wk; Kabi-Pharmacia, Stockholm, Sweden) administered subcutaneously improve linear growth in children with CRI68 and those undergoing peritoneal dialysis69 or HD during the first year of treatment.70 However, the magnitude of improvement in linear growth in patients treated with dialysis is not as great as that observed in children with stable CRF.71,72 Furthermore, the gain in height during subsequent years of recombinant hGH treatment is diminished.69,70 Therefore, the efficacy of long-term recombinant hGH therapy remains to be established in children receiving MD.
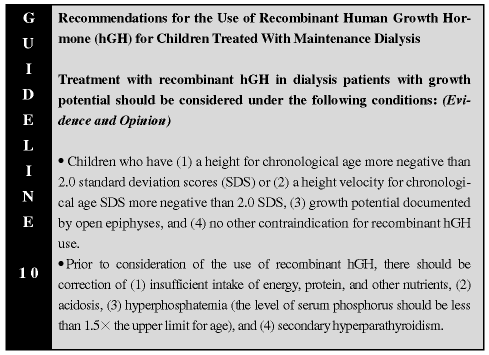
A lack of response to recombinant hGH therapy has been seen with suboptimal energy or protein intakes or in children with metabolic acidemia. Correction of these abnormalities is essential before initiation of recombinant hGH therapy. A serum bicarbonate value below 22 mmol/L requires exogenous alkali therapy (see Guideline 2). An expected effect of recombinant hGH therapy is to increase intact parathyroid hormone (PTH) levels in the first 6 months of therapy.73 Therefore, to prevent potentially deleterious effects of worsening secondary hyperparathyroidism in children, attempts to control elevated serum PTH levels (intact assay values less than 500 pg/mL; normal range, 10 to 55 pg/mL) is necessary prior to initiation of recombinant hGH therapy. Additionally, it is suggested that monitoring of intact PTH levels be performed at least every 3 months during the first 6 months of recombinant hGH therapy in these children. Severe hyperphosphatemia also impairs the action of recombinant hGH, and it is important to maximize the control of serum phosphorus levels in these patients prior to initiating treatment with recombinant hGH.
If the patient does not respond to recombinant hGH after 12 months of treatment, discontinuation of recombinant hGH should be considered. A lack of response to recombinant hGH is defined as gain of growth velocity by less than or equal to 2 cm compared with that observed during the previous year. Prior to discontinuation of recombinant hGH therapy, a thorough evaluation of the patient should be undertaken to assure that other causes that contribute to growth retardation in children with CRF have been corrected. Continuation of recombinant hGH at this point would depend on correction of these other factors.
If the patient reaches the 50th percentile for target height following recombinant hGH treatment, it is advisable to discontinue recombinant hGH treatment and monitor the patient. If the height SDS decreases by 0.25 during a subsequent observation period, it is advisable to consider reinstitution of recombinant hGH therapy.