See Appendix 2.
Poor energy intake is common in children with CKD stages 2 to 5 and 5D due to reduced appetite and vomiting. Early intervention is critical with the introduction of tube feeds if energy requirements cannot be met by the oral route alone. A smaller percentage of children have excessive energy intake, and dietary intervention and lifestyle changes are needed to address the short- and long-term complications of overweight and obesity.
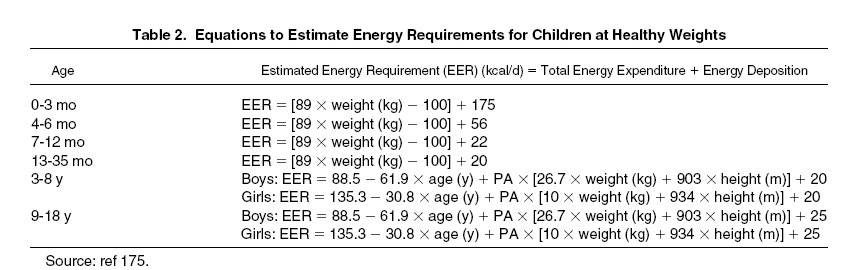
4.1 Energy requirements for children with CKD stages 2 to 5 and 5D should be considered to be 100% of the EER for chronological age, individually adjusted for PAL and body size (ie, BMI). (B) Further adjustment to energy intake is suggested based upon the response in rate of weight gain or loss. (B)
4.2 Supplemental nutritional support should be considered when the usual intake of a child with CKD stages 2 to 5 or 5D fails to meet his or her energy requirements and the child is not achieving expected rates of weight gain and/or growth for age. (B)
4.3 Oral intake of an energy-dense diet and commercial nutritional supplements should be considered the preferred route for supplemental nutritional support for children with CKD stages 2 to 5 and 5D. (B) When energy requirements cannot be met with oral supplementation, tube feeding should be considered. (B)
4.4 A trial of intradialytic parenteral nutrition (IDPN) to augment inadequate nutritional intake is suggested for malnourished children (BMI-for-height-age < 5th percentile) receiving maintenance HD who are unable to meet their nutritional requirements through oral and tube feeding. (C)
4.5 A balance of calories from carbohydrate and unsaturated fats within the physiological ranges recommended as the AMDR of the DRI is suggested when prescribing oral, enteral, or parenteral energy supplementation to children with CKD stages 2 to 5 and 5D. (C)
4.6 Dietary and lifestyle changes are suggested to achieve weight control in overweight or obese children with CKD stages 2 to 5 and 5D. (C)
4.1: Energy requirements for children with CKD stages 2 to 5 and 5D should be considered to be 100% of the EER for chronological age, individually adjusted for PAL and body size (ie, BMI). Further adjustment to energy intake is suggested based upon the response in rate of weight gain or loss. (B)
In children with CKD (excluding CKD stage 5), spontaneous energy intake decreases with deteriorating kidney function,28 but there is no evidence that children with CKD have different energy requirements than those for healthy children. In a recent study of 25 children and adolescents with CKD stage 5 on HD therapy, resting energy expenditure measured by using indirect calorimetry was the same as for healthy age-matched controls when adjusted for lean body mass.174 In 65 children aged 2 to 16 years with conservatively managed CKD (GFR < 75 mL/min/1.73 m2), regular dietetic advice with particular attention to optimizing energy intake with or without the use of supplements maintained or significantly increased the height SDS with an energy intake maintained within the normal range.156 In 35 children younger than 5 years with CKD stages 4 to 5, significant weight gain and accelerated linear growth was clearly demonstrated in those starting enteral feeding at age younger than 2 years; improved weight gain and maintenance of growth was observed in those starting enteral feeds at age 2 to 5 years without exceeding normal energy requirements.18 The findings are similar to an earlier study of 22 children age 0.2 to 10 years on long-term dialysis therapy in which there was significant improvement in both height and weight SDS with an energy intake within the normal range.154 Improved linear growth also has been demonstrated in 12 prepubertal or early pubertal children on HD therapy with increased time on dialysis and close monitoring of nutritional intake. This was achieved with an intake of 90.6% of the recommended energy intake.150 The importance of caloric intake has also been shown in 31 prepubertal children on dialysis therapy treated with growth hormone, with a positive correlation between energy intake and growth velocity.26
All children with CKD stages 2 to 5 and 5D should have regular dietary assessments, with the frequency dependent on the degree of renal impairment to ensure EER for age, sex, and PAL (Tables 2 to 4; Appendix 2, Table 34 for online calculator) are achieved. If children younger than 3 years with a length- or height-for-age less than −1.88 SDS fail to achieve expected weight gain and growth when receiving EER (Table 2) based on chronological age, estimated requirements may be modified by using height-age.

See Appendix 2.
As in the general public, the incidence of childhood obesity in those with CKD is increasing. National registry data for pediatric dialysis or transplant patients showed a significantly higher mortality rate at the upper and lower extremes of BMI-for-age.49 Pretransplantation obesity is associated with decreased long-term renal allograft survival.176 Prevention and treatment of obesity in patients with CKD is also important to reduce the risk of hyperlipidemia. Fat mass is less metabolically active than lean mass; therefore, energy requirements for overweight or obese children are lower and can be estimated by using equations specific for children heavier than a healthy weight (Table 3).
In infancy, feeds should be of breast milk or a whey-based infant formula with a low renal solute load if needed. Weaning solids should be introduced at the same time as recommended for healthy children. In children, high-energy foods and drinks are recommended as part of a controlled intake, with nutritional supplements or nutritionally complete feeds introduced if necessary. Calculated energy requirements are estimates, and some children will require more or less for normal growth; therefore, all dietary prescriptions should be individualized.
Early intervention to try to prevent the development of oral hypersensitivity and food-aversive behavior often is incorporated into the feeding plan and includes the correct timing for introduction of solids with gradual inclusion of new tastes and lumpier textures, messy play and food exploration, prohibition of force feeding with self-feeding behavior promoted, and sitting with the family at meal times.
Other members of the multidisciplinary team with expertise in infant feeding issues—eg, infant psychologists and speech, language, and occupational therapists—may be important in improving the outcome for normal feeding. However, overemphasis on maintaining the oral route to achieve an adequate nutritional intake may be counterproductive because symptoms may be exacerbated by inappropriate expectations and the critical period of intervention to ensure normal nutrition dependent growth may be missed.
In children with CKD stage 5D on PD therapy, variable glucose absorption takes place from the dialysis fluid depending on the mode of dialysis, dialysate glucose concentration, and peritoneal membrane capacity. There are 2 adult studies documenting the caloric impact from dialysis fluid glucose.177,178 One formula using both PD modality and peritoneal equilibration test (PET) transport characteristics was shown to closely approximate measured glucose absorption, but has not been evaluated in children.177 In a pediatric study of 31 children older than 3 years on ambulatory PD therapy, the mean energy intake derived from peritoneal glucose absorption was 9 kcal/kg/d.152 Kaiser et al136 demonstrated better growth rates in children receiving CAPD versus CCPD versus HD that may have been partially explained by increased glucose absorption associated with CAPD. Because many children on PD therapy are underweight, the prescribed energy intake in those with CKD stage 5D should exclude the estimated calorie absorption from the dialysate because this may compromise the nutritional quality of the diet. However, some children—and particularly infants on PD therapy—gain weight at a faster rate than normal despite oral and/or enteral energy intakes that are lower than the average requirements. Reduced physical activity and increased exposure to dialysate glucose for fluid removal may be explanations, and in these cases, the calorie contribution from PD fluid should be taken into account when estimating energy requirements.
4.2: Supplemental nutritional support should be considered when the usual intake of a child with CKD stages 2 to 5 or 5D fails to meet his or her energy requirements and the child is not achieving expected rates of weight gain and/or growth for age. (B)
4.3: Oral intake of an energy-dense diet and commercial nutritional supplements should be considered the preferred route for supplemental nutritional support for children with CKD stages 2 to 5 and 5D. (B) When energy requirements cannot be met with oral supplementation, tube feeding should be considered. (B)
Energy requirements in infants and children include the energy needed for tissue deposition, with satisfactory growth a sensitive indicator of whether energy requirements are being met, particularly in infancy.179 Poor energy intake and vomiting in children with CKD therefore will have an adverse effect on growth. Because short stature at dialysis therapy initiation is a marker for poor outcome in children initiating dialysis therapy, early intervention with intensive nutritional support may be critical to outcome.180 Because calculated energy requirements are estimates, all dietary prescriptions should be individualized because some children will require more or less for normal growth. Formulas and enteral feedings may be concentrated and/or supplemented with a commercial glucose polymer powder and/or a liquid fat. Energy-dense feeds may be needed in children with CKD stage 5 with oligoanuria (see Tables 2 to 4 for EER; Appendix 2, Table 34, for resources to calculate EER; and Appendix 3, Table 36, for information for feeds and supplements).
However, both poor appetite and vomiting are common in infants and children with CKD and have a negative impact on the aim of achieving the dietary prescription. Poor appetite is multifactorial in origin and includes a thirst for water rather than feed in those with polyuric CKD, the administration of multiple unpleasant medications, and a preference for salty rather than energy-dense sweetened foods. The accumulation of appetite-regulating cytokines and hormones has been implicated in the cause of both this lack of spontaneous appetite and early satiety and provides a physiological explanation for the difficulties faced by caregivers in delivering the dietary prescription.181,182 Gastroesophageal reflux was demonstrated in 73% of infants with chronic kidney failure, with poor feed intake and vomiting183 and disordered gastric motility, delayed gastric emptying, and gastroesophageal reflux in 12 symptomatic children in association with increased polypeptide hormone levels.184
Symptoms of vomiting, irritability, and discomfort suggestive of gastroesophageal reflux initially should be managed conservatively by concentrating feeds to reduce feed volume and minimizing seated and supine positions after feeds because there is some evidence of benefit in infants without CKD.185,186 Although there are no published data about the use of prokinetic agents (eg, metoclopramide, a dopamine receptor antagonist; domperidone, a peripheral D2 dopamine receptor antagonist) or gastric acid suppressants (H2 receptor blockers or proton pump inhibitors) in children with CKD, their use may be helpful. If symptoms persist, anatomic abnormalities should be excluded radiologically, but the role of routine pH studies and tests of gastric emptying in those with CKD is not established. A fundoplication may be indicated for intractable vomiting and can be performed after a gastrostomy is placed.
When poor appetite and vomiting preclude a nutritionally adequate intake, tube feeding commonly is implemented. Although registry data from the North American Pediatric Renal Transplant Cooperative Study (NAPRTCS) for the use of supplemental tube feeds in children younger than 6 years at the start of dialysis therapy showed no improvement in linear growth, follow-up was for only a year and no information was available for calorie intake.187 However, in single-center studies, tube feeding has been shown to facilitate weight gain and growth. Significant weight gain and catch-up growth were achieved in 35 children with CKD stages 4 to 5 and age younger than 5 years if tube feeding was started before the age of 2 years. Loss of nutrition from vomiting is variable and hard to assess; however, the improved weight gain observed in this study over 2 years with enteral feeding and without an increase in energy intake for age suggests that vomiting can be reduced by slow delivery of feeds.18 In a large study of 101 infants presenting with CKD who were younger than 6 months and had a GFR less than 20 mL/min/1.73 m2 or CKD stage 5 within 2 years, 81% of the 81 survivors were tube fed and achieved a mean height SDS within the normal range by 1 year, with continued improvement thereafter.17 In 12 infants starting PD therapy at younger than 1 year and on PD therapy for at least a year in association with enteral feeding, height, weight, and occipital head circumference SDS all improved significantly by 1 year, with continuing improvement in weight and occipital head circumference into the second year.188 Coleman et al154 included older children in their study of tube feeding using gastrostomy buttons in 22 children (0.2 to 10.3 years old) on long-term dialysis therapy. Although growth data did not distinguish between those starting gastrostomy feeding before (n = 16) or after the age of 5 years (n = 6), mean height and weight SDS increased significantly by 18 months. However Ramage et al,189 in a study of 15 children on PD therapy and gastrostomy fed, subdivided growth outcome into those age younger than 2.5 years (n = 8) and those older than 2.5 years (n = 7) at the start of tube feeding. There was no further decrease in height SDS in either group, with significant weight gain in both age groups by 12 months.189 Therefore, tube feeding should be considered for infants and children younger than 3 years who do not meet their EER orally despite dietary intervention and who are underweight or growth retarded (weight or length/height < −1.88 SDS) or failing to achieve normal rates of weight gain or growth. Although there are limited data about the use of tube feeding in children older than 3 years, this approach should be considered in the individual child with intake inadequate to maintain expected weight gain to prevent malnutrition, which increases the risk of infection, reduces stamina and cognition, and compromises long-term survival.70 However, treatment with growth hormone may be indicated if growth failure persists despite meeting nutritional requirements, particularly after early childhood, because there is currently minimal evidence that improved nutrition alone can facilitate catch-up growth.
The method of tube-feed delivery and feed composition will depend on age, the presence or absence of vomiting, nutrient requirements, mineral and electrolyte imbalances, and the assessed intake that can be achieved orally. Infants may require only boluses of the balance of their feeding after oral feeds (ie, top-up boluses), but some may need the full prescription to be given by tube, which can then be delivered by pump as an overnight feed, with the rate adjusted as tolerated with additional daytime boluses (see Appendix 4, Table 38 for information about introducing and advancing enteral feeds). Older children may benefit from having the majority of their feed overnight to encourage hunger and oral intake during the day and so they can be free to undertake normal daytime activities without the pressure to meet all their requirements while at school or socializing.
Dello Strogolo et al168 reported persistent feeding dysfunction in 8 of 12 infants with a GFR less than 35 mL/min/1.73 m2 who were managed with nasogastric tube feeds for at least 9 months. Therefore, it is important that tube-fed infants and children be encouraged to continue some oral intake or have continued oral stimulation, eg, sucking on a pacifier and/or positive nonthreatening contact with food. Other studies are more encouraging. In 5 infants on PD therapy and nasogastric feeding with persistent food refusal, intensive behavior therapy by a multidisciplinary team enabled the infants to convert to full oral feeding.190 Although there are concerns that tube feeding will further reduce oral intake, Ledermann et al18 showed in children aged 0 to 2 years that the percentage of energy derived from the tube feed did not change over 2 years despite an increase in the absolute energy intake with age, confirming improved oral intake. The long-term outlook for normal feeding after transplantation is excellent, with reports of successful transitioning of almost all tube-fed children to oral diet and fluids within 10 months if children with significant comorbidity are excluded.169,191
Although the preferred method of tube feeding is by means of gastrostomy, nasogastric tubes may be used long term or as a temporary measure, particularly for infants weighing less than 4 kg or infants/children presenting with CKD stage 5 needing immediate PD therapy. Repeated replacement due to vomiting with subsequent aversive behavior and the psychosocial problems associated with the visibility of the tube are averted by the use of gastrostomies. Gastrostomies may be placed either percutaneously (radiologically or endoscopically) or by using an open procedure. Minor complications are well documented for both approaches, particularly exit-site erythema and infections. Migration of the retention disk and enterocolic fistulae can present as significant late complications of percutaneous placement, although the latter may be avoided by radiological placement because the bowel is outlined with contrast. Gastrocutaneous fistulae may need surgical closure after gastrostomy button removal. A percutaneously placed gastrostomy should be replaced every 18 to 24 months by either the same size gastrostomy tube or, if the track is adequate, a button gastrostomy according to the child's and family's preference.192,193 Ideally, placement of a gastrostomy tube should occur before PD catheter placement. The placement of a percutaneous gastrostomy while on PD therapy should be discouraged because the risk of severe peritonitis and PD failure is high; conversely, an open Stamm gastrostomy, initially with a catheter and subsequently replaced by a button device, can be performed safely in children on PD therapy with suitable precautions (eg, antibiotic and antifungal coverage and time off PD therapy after placement). There is no evidence of an increased incidence of bacterial or fungal peritonitis with an established gastrostomy.155,194,195
A fundoplication may be performed with the gastrostomy or after initial gastrostomy placement if severe vomiting persists despite medical and nutritional management, but temporary HD therapy may be required.155,196 A Stamm gastrostomy can be created at the same time as PD catheter placement without additional complications.154
The use of gastrojejunal tubes has been described by Geary and Chait,110 but the expected reduction in vomiting was not observed and the need for continuous feed delivery reduces the practical application.
Other approaches may improve the nutritional status. In adult maintenance HD patients, increasing dialysis frequency to 6 times/wk improved both biochemical markers and weight gain.197 A recent report of increased growth velocity in 5 children with intensified daily HD allowed a "free" diet raises the possibility that nutritional status improves with a higher dialysis dose.149 Although the appetite stimulant megestrol acetate has been used in adults on HD therapy,198,199 there are significant side effects and no published studies or case reports of the use of appetite stimulants or anabolic agents in children with CKD.
The 3½- to 4-hour HD session, which characteristically occurs thrice weekly, may offer an opportune time to provide oral nutritional supplementation, provided the patient is tolerant of the nutrient intake during the session. Although this is a common practice in Europe, the experience in many other centers has been less positive, prompting a philosophy against the allowance of oral intake during HD in adult and even pediatric centers alike.200-203 The most frequent adverse outcome noted when meals have been provided is hypotension, presumably the result of either decreased cardiac output secondary to splanchnic sequestration of blood or through a decrease in splanchnic resistance leading to a reduction in systemic vascular resistance.204,205 A decrease in relative blood volume also has been documented.206 However, more recently, a prospective study of 85 adults receiving maintenance HD revealed the nutritional benefit and patient tolerance of an oral supplement provided during the HD session.207 In a subsequent retrospective study of 126 stable adult HD patients, there also was no evidence of an association between oral intake during HD and intradialytic hypotension, although the prescribed dry weight was not achieved in a substantial percentage of patients with high oral intake.208 It is distinctly possible that the fewer comorbidities that characterize pediatric versus adult patients receiving HD are associated with decreased risk of postprandial complications. However, evidence supporting this hypothesis is not yet available and mandates close monitoring of vital signs in any patient who receives nutritional supplementation during an HD session.
4.4: A trial of IDPN to augment inadequate nutritional intake is suggested for malnourished children (BMI-for-height-age < 5th percentile) receiving maintenance HD who are unable to meet their nutritional requirements through oral and tube feeding. (C)
Malnutrition, short stature, and low BMI are independent risk factors for mortality in adult and pediatric patients.49,70,209 Data from adult patients receiving maintenance HD show that anorexia is an independent risk factor for death 12 months later.210 Children receiving maintenance dialysis report high rates of depression,211 poor adjustment to diagnosis and lower socioeconomic status,212 and lower health-related quality of life213-215 than healthy controls and therefore are at risk of anorexia-induced malnutrition. One pediatric center reports that psychosocial/malnutrition-related causes account for the most frequent reason for HD patient hospitalization.58 Advanced CKD stages are often associated with anorexia and gastrointestinal disorders, which may inhibit the ability to maintain adequate nutritional status through the oral and/or enteral route. IDPN can be provided to augment inadequate nutritional intake in a small select group of children who are malnourished and unable to meet their requirements through oral and tube feeding.
Pilot pediatric data from small cohorts suggest that IDPN can be efficacious to augment inadequate oral and/or enteral nutrition in malnourished children, leading to improvements in BMI in children with organic,58,59,216 but not psychosocial,59 causes of malnutrition. Optimal IDPN solution composition is unknown; however, a typical IDPN prescription contains amino acids in amounts to meet estimated daily protein requirements, as well as dextrose and 20% or 30% lipid components to increase the caloric impact of the IDPN. Substrate infusion rates are adjusted upward as tolerated to enhance caloric intake while preventing or managing hyperglycemia and hyperlipidemia (Table 5) .
Although data assessing IDPN efficacy in adult HD patients have not shown a clear benefit of IDPN to reduce mortality,217,218 such data may not be applicable to children, for whom adequate nutrition is requisite for normal growth and development.
IDPN is administered continuously during the entire course of the HD treatment and should be infused in the venous limb of the HD circuit to prevent clearance of amino acids and trace elements. More than two-thirds of the infused amino acids are retained, and the fluid used to deliver IDPN is removed through ultrafiltration. Trace element solutions can be added to provide zinc, copper, selenium, manganese, and chromium. Table 6 lists the potential adverse events associated with IDPN and a recommended monitoring schedule. Postinfusion hypoglycemia or symptoms suggestive of refeeding syndrome (eg, hypokalemia, hypophosphatemia, and hypomagnesemia) have been seen rarely in children on IDPN therapy.
In the absence of pediatric criteria, discontinuation criteria for adults may provide guidance.217,219 Suggested criteria include clinical evidence of improving nutrition as evidenced by increased dry weight and an increase in oral intake to meet energy and protein requirements. Additional criteria for discontinuation include no improvement in nutritional status after 4 to 6 months of IDPN or complications or intolerance of IDPN therapy.219
IDPN provision can require substantial resources and should be used only when adequate financial and personnel resources are available. IDPN should not be promoted as a sole nutrition source; it should be used to augment other sources. If the combination of oral and/or enteral intake and IDPN is unable to meet energy and protein requirements, daily total or partial parenteral nutrition is indicated.
4.5: A balance of calories from carbohydrate and unsaturated fats within the physiological ranges recommended as the AMDR of the DRI is suggested when prescribing oral, enteral, or parenteral energy supplementation to children with CKD stages 2 to 5 and 5D. (C)
Fats, carbohydrates, and proteins can substitute for one another to some extent to meet the body's energy needs. Uneven distribution of calories from each of the macronutrients may be associated with inadequacy of certain nutrients and increased risk of such chronic diseases as coronary heart disease, obesity, and diabetes. Cardiovascular disease (CVD) is the leading cause of morbidity and death in the pediatric CKD population.220,221 Upper extremes of BMI-for-age are associated with higher mortality rates in children on dialysis therapy and decreased long-term allograft survival and higher mortality rates in pediatric transplant patients. Although large-scale studies of risk-factor outcomes for those with CVD have not been performed in adults or children with CKD, the high mortality rate supports the need for risk-factor reduction early in the course of CKD to reduce long-term exposure to cardiovascular insult and improve outcomes. To achieve the best risk reduction, it appears that dietary strategies should aim to prevent or minimize increased triglyceride (TG) and cholesterol levels and avoid conditions—such as obesity—that contribute to dyslipidemia.
It often is necessary to supplement an infant's formula or a child's diet with fat and carbohydrate to provide optimal calories, especially when the child is fluid restricted. In the general population, low or high proportions of calories from carbohydrate or fat are associated with nutrient inadequacies (eg, fat-soluble vitamins) and/or chronic diseases, including heart disease, obesity, and diabetes.175
Macronutrients are related to heart disease and obesity in many ways. Excess energy intake results directly in obesity, which increases the risk of heart disease. High intake of dietary cholesterol, saturated fat, or trans fatty acids can increase total and low-density lipoprotein (LDL) cholesterol levels in the blood whereas monounsaturated and polyunsaturated fatty acids a decrease total and LDL blood cholesterol levels. High intakes of n-3 polyunsaturated fatty acids (omega-3 fatty acids [n-3 FA], docosahexanoic acid [DHA], and eicosapentanoic acid [EPA]) are associated with decreasing TG levels and a decreased risk of heart disease. High carbohydrate (ie, simple sugars) and low fat intakes tend to increase plasma TG levels and decrease high-density lipoprotein (HDL) cholesterol levels, with a carbohydrate source of monosaccharides (especially fructose) causing a more extreme effect. Hypertriglyceridemia also has been associated with enhanced glucose uptake in children on PD therapy. Dietary fiber, particularly naturally occurring viscous fiber, reduces total and LDL cholesterol levels, and high intakes have been associated with reduced rates of CVD.
As noted previously, CVD is the leading cause of morbidity and mortality in children with CKD, accounting for approximately 25% of total deaths.220,221 These rates are 1,000 times higher than the national pediatric cardiovascular death rate.220 CVD in children with CKD is associated with traditional (dyslipidemia, hypertension, obesity, physical inactivity, and genetics) and nontraditional factors (uremia, uremia-related anemia, prothrombogenic factors, inflammation, fluid overload, left ventricular hypertrophy, increased homocysteine levels, and vascular calcification).220 Children with CKD have been identified as being in the highest risk category for pediatric CVD.173
Dyslipidemia occurs relatively early in the progression of CKD (ie, GFR, 30 to 59 mL/min/1.73 m2) and increases in prevalence as kidney function deteriorates.222 In children and adolescents on PD therapy, reported rates of dyslipidemia range from 29% to 87%.223 Hypertriglyceridemia and hypercholesterolemia have been reported in 90% and 69% of children with CKD stage 5, respectively.224 Dyslipidemia in pediatric CKD manifests primarily as increased levels of serum TG, contained predominantly in very LDLs (VLDLs) of hepatic origin.225 This occurs in combination with high levels of VLDL and intermediate-density lipoproteins (IDLs), low levels of HDL particles, and normal or modestly increased levels of total and LDL cholesterol.226,227 Sometimes referred to as atherogenic dyslipidemia, the metabolic abnormalities underlying it are complex.227 Hypertriglyceridemia is an independent contributor to the development of CVD228-232 and may also accelerate progression of CKD to CKD stage 5, dialysis, and transplantation.233-235
Recommended ranges for a healthy distribution of calories from protein, fat, and carbohydrate for the general pediatric population have been established by the DRI.175 These AMDR (Table 7) are based on evidence that consumption greater or less than these ranges may be associated with nutrient inadequacy and increased risk of developing such chronic diseases as coronary heart disease, obesity, diabetes, and/or cancer. There is no information to suggest that dietary advice regarding macronutrient distribution in children with CKD should be different from that in the general population; therefore, it seems prudent to maintain a distribution of calories similar to that recommended by the AMDR for children with CKD stages 2 to 5 and 5D.
The DRI provide further recommendations for specific types of carbohydrate and fat to avoid or limit for the purpose of chronic disease risk reduction (Table 8). Given the high risk of CVD in children with CKD, it is recommended that children and their caregivers be counseled to use sources of unsaturated fat rather than saturated or trans fats and, as much as possible, to choose complex carbohydrates instead of simple sugars.
Calorically dense formulas frequently are prescribed for infants; however, there are no AMDR for those younger than 1 year. Therefore, when advancing the caloric density of formula, the distribution of protein, fat, and carbohydrate should be kept consistent with the base formula,236 which must adhere to strict standards (7% to 12% protein, 40% to 54% fat, and 36% to 56% carbohydrate; www.codexalimentarius.net; last accessed March 30, 2008). Infants and young children need a somewhat greater proportion of fat in their diets to meet energy needs. Protein and electrolyte issues typically predict whether the energy density of an infant's formula can be concentrated (ie, more formula concentrate and less water) or increased by the addition of modular components of carbohydrates (eg, powder or liquid forms of tasteless glucose polymers) and/or fat (eg, ordinary oil used at home, emulsified oil, or medium-chain TG; Appendix 3, Table 36). When uremia, hyperkalemia, hyperphosphatemia, or formula osmolarity prevent concentrating formulas, additions of carbohydrate and/or fat are indicated. Fat additions to formula should be in the form of heart-healthy unsaturated fats, such as canola, olive, or corn oil. Providing enteral feedings containing glucose polymers and oil emulsions in a balanced profile of fat and carbohydrate to children with CKD managed conservatively (n = 5) or by using PD (n = 5) did not enhance hyperlipidemia compared with 37 children who were not tube fed.237
Children with CKD stages 2 to 5 and 5D and dyslipidemia have been identified as a high-risk population for CVD.173 Table 9 lists more precise recommendations for stricter lowering of total dietary fat, cholesterol, and trans and saturated fats directed to toddlers, children, and adolescents with dyslipidemia and CKD stage 5, 5D, or a kidney transplant.
The K/DOQI Dyslipidemia Guidelines' recommendations, endorsed by the K/DOQI Cardiovascular Guidelines, recommend that the dietary and lifestyle recommendations made for adults are also appropriate for postpubertal children and adolescents with CKD (Table 11), but that prepubertal children should follow recommendations from the National Cholesterol Expert Panel in Children and Adolescents (NCEP-C).238 Since then, a consensus statement on dietary recommendations for children and adolescents from the American Heart Association (AHA),239 endorsed by the American Academy of Pediatrics, provides more current guidance than the NCEP-C recommendations for working with children and adolescents with CKD (Tables 9 and 10), recognizing that dietary modifications to increase calories or restrict potassium and/or phosphorus intake make macronutrient modifications more challenging to achieve.
The extent to which the macronutrient content of the diet should be manipulated must consider the child's nutritional status and other dietary mineral and/or electrolyte restrictions. The first priority for nutritional care is meeting energy, protein, and micronutrient requirements to achieve optimal growth for individual children. If a child is well nourished, adding dietary modifications for dyslipidemia prevention or management can be safely undertaken. Studies of the general pediatric population have shown that dietary fat restriction to 30% of total caloric intake is safe and, in particular, free of adverse effects on growth, development, or nutrition.240,241
Renal diet restrictions to control uremia (protein) and mineral and electrolyte abnormalities limit the variety and palatability of the diet, and additional (dyslipidemia) restrictions can be overwhelming and may reduce caloric intake further. In light of this, dietary intervention for treatment of dyslipidemia is not recommended for undernourished children with CKD220,223; however, such simple changes as a switch to heart-healthy fats can be implemented easily.
4.6: Dietary and lifestyle changes are suggested to achieve weight control in overweight or obese children with CKD stages 2 to 5 and 5D. (C)
Childhood obesity is an international public health problem reaching epidemic proportions. A review of data from the US Renal Data System for more than 1,900 pediatric dialysis or transplant patients showed that mortality rates were significantly higher at the upper and lower extremes of BMI-for-age.49 Pretransplantation obesity and increased BMI-for-age after transplantation are associated with decreased long-term renal allograft survival.176 Prevention and treatment of obesity in patients with CKD is also important to reduce the risk of hyperlipidemia.242
A multiorganization scientific statement on cardiovascular risk reduction in high-risk pediatric patients made the following recommendations for high-risk children, including those with CKD stages 5 and 5D and kidney transplant recipients with a BMI greater than the 95th percentile. Step 1 treatment: (a) age-appropriate reduced-calorie training for child and family; (b) specific diet/weight follow-up every 4 to 6 months, repeated BMI calculation at 6 months; and (c) activity counseling with a goal of 1 hour or more of active play per day and screen time limited to 1 hour or less per day. Step 2 treatment if follow-up BMI remains greater than the 95th percentile: weight-loss program referral plus consider referral for exercise testing and recommendations from exercise specialist appropriate for cardiac status.173 Interventional strategies for treatment of child and adolescent overweight and obesity in the non-CKD population45 may be helpful.
The AI for total fiber is based on daily caloric intake, and for all children 1 year and older is 14 g/1,000 kcal/d. To normalize cholesterol levels and reduce the risk of cardiovascular heart disease, an increase in soluble fiber intake is recommended as an addition to reductions in saturated fatty acid and cholesterol intake.239,241 Fiber also can aid laxation and promote satiety, which can reduce energy intake and the risk of overweight.
Dietary fiber is found in most fruits, vegetables, legumes, and whole grains, which are foods restricted in low-potassium and low-phosphorus diets; therefore, meeting daily fiber recommendations for healthy children is more challenging for children with CKD who have limited intake of these foods due to low-potassium and/or low-phosphorus diet restrictions. Appendix 3, Table 37 lists some foods containing 1.9 g or greater of fiber per serving and includes their potassium and phosphorus content to guide advice about increasing fiber intake for individual children. High-fiber foods with extremely high potassium and/or phosphorus content have been omitted. Tasteless mineral- and electrolyte-free powdered forms of fiber (eg, Unifiber®, Benefiber®) are available to add to meals or drinks if children are unable to meet their fiber intake by diet. High-fiber diets require additional fluid intake, which may not be possible for oliguric or anuric patients with strict fluid restriction.
Approximately 75% of children with CKD have hypertriglyceridemia, for which there is no effective therapy. Both primary and secondary prevention studies provide strong evidence that consumption of fish and fish oils rich in the n-3 FAs EPA and DHA reduce all-cause mortality and various CVD outcomes in adults.243,244 By far, the strongest most consistent evidence of the cardioprotective benefits of n-3 FA is for the lowering of serum TG levels that is dose dependent and similar in various (adult) populations.244,245 Adults with CKD who were treated with n-3 FA for 8 weeks had significant decreases in TG levels ranging from 20% to 50% compared with controls.246-248 Pediatric data for the TG-lowering effect of n-3 FA are limited to several pre/post studies.249,250 Eighteen children (7 to 18 years old) on dialysis therapy experienced a 27% decrease in TG levels from 236 ± 31 to 171 ± 21 mg/dL after 8 weeks of EPA plus DHA supplementation.251 In a trial of n-3 FA and alternate-day prednisone on progression of disease in children and young adults (age, 7.4 to 39.7 years) with immunoglobulin A (IgA) nephropathy, a 17% decrease in TG level was observed after 2 years of therapy with 3.36 g/d of EPA plus DHA.252
EPA and DHA can be synthesized in vivo through the elongation and desaturation of α-linolenic acid; however, this process occurs slowly and is inefficient. Therefore, EPA and DHA, found almost exclusively in fish and marine sources, must be provided in the diet; the highest sources are fatty fish (eg, tuna, mackerel, trout, salmon, herring, sardines, and anchovies).253 Adults on dialysis therapy consume fish in amounts far less than recommendations and have lower tissue EPA plus DHA stores compared with healthy people.254 The higher mercury content of certain fatty fish (shark, swordfish, marlin, orange roughy, king mackerel, escolar [snake mackerel], tilefish, and albacore or "white" tuna) has led various regulatory bodies to issue recommendations about the maximum intake of these fish for young children, who are considered to be more susceptible than adults to the adverse health effects of methylmercury.
Several safety concerns around the use of n-3 FA have been raised, including prolonged bleeding times, worsening glycemic control in patients with diabetes, small increases in LDL cholesterol levels, and environmental contaminants in fish-oil products. Despite these concerns, n-3 FAs have been found to be extremely safe by both Health Canada and the US Food and Drug Administration.
At this time, there is insufficient evidence to recommend routine use of n-3 FAs to treat hypertriglyceridemia in children with CKD.