9.1 In the opinion of the Work Group, there is currently insufficient evidence to suggest a role for carnitine therapy in children with CKD stage 5D.
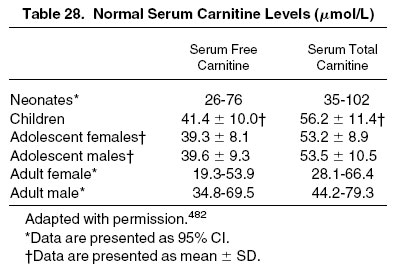
L-Carnitine is a biologically active amino acid derivative that has a key role in the regulation of fatty acid metabolism and adenosine triphosphate formation in multiple organs.481 Total carnitine concentration includes both free and bound (ie, acylated) carnitine, which reflects levels in both serum and tissues, such as muscle, liver, and kidney. Total acylcarnitine levels are increased in patients with CKD stage 5D, with values as much as 4.6 times greater than in healthy subjects.481 Carnitine deficiency is confirmed by measurements of plasma free and total carnitine with an acyl:free carnitine ratio greater than 0.4 (ie, [total − free carnitine] ÷ free carnitine) or a total serum carnitine value less than 40 μmol/L (Table 28).482
Patients who receive HD may be at risk of the development of carnitine deficiency as a result of loss of carnitine during the dialysis procedure, in addition to possible reductions in dietary intake and endogenous synthesis. In turn, patients on HD therapy have been documented to have low plasma and tissue L-carnitine levels.481-484 Far less information pertaining to the relationship between dialysis and carnitine deficiency is available from the PD population.485,486
Carnitine deficiency can result in the development of anemia, cardiomyopathy, and muscle weakness, all symptoms that may be present in the dialysis population.481,482,487,488 It is also associated with intradialytic hypotension in patients receiving HD. However, studies addressing the therapeutic use of supplemental L-carnitine in dialysis patients are few and have characteristically included small numbers of patients.485,486,489,490 This has compromised any ability to generate definitive evidence supporting the role of regular supplemental L-carnitine in the treatment of these symptoms. Along these lines, the KDOQI Adult and Pediatric Work Group on Anemia Management conducted a thorough evaluation of the data particular to the treatment of anemia in patients with CKD and concluded there was insufficient evidence to recommend a role for carnitine in the treatment of anemia.491 Most, but not all, of the few pediatric studies that have been conducted on the subject of carnitine deficiency in dialysis patients have provided evidence for an increase in plasma carnitine level after carnitine supplementation with no associated change in any symptoms.485,486,489,490
Although the Work Group cannot recommend the use of carnitine at this time, it does not want to discourage any therapeutic trial of carnitine if the clinical symptoms are suggestive of the disorder, especially when the evaluation provides laboratory evidence compatible with a diagnosis of carnitine deficiency. In a manner similar to that of an NKF Carnitine Consensus Conference and in line with the recommendation from the prior K/DOQI Pediatric Nutrition Guidelines, the Work Group believes that a trial may be indicated when all other causes for the symptoms in question have been excluded and the patient has been unresponsive to standard therapies.487 Although carnitine supplementation has been provided through the intravenous and oral routes in patients with CKD, there have not been comparative studies of the 2 routes of therapy despite significant differences in their respective pharmacokinetics.485,486,490 The bioavailability of oral L-carnitine in patients with CKD is unknown. In studies of healthy adults, the portion of oral L-carnitine that is not absorbed is converted to trimethylamine-N-oxide and trimethylamine, metabolites normally excreted by the kidney. Accumulation of these metabolites and their breakdown products in patients with CKD may be associated with the development of neurotoxicity and “uremic breath.” 481,487,492