12.1 To prevent aluminum toxicity, the regular administration of aluminum should be avoided and the dialysate concentration of aluminum should be maintained at <10 µg/L. (EVIDENCE)
12.1.a CKD patients ingesting aluminum should not receive citrate salts simultaneously. (EVIDENCE)
12.2 In CKD Stage 5, to assess aluminum exposure and the risk of aluminum toxicity, serum aluminum levels should be measured at least yearly and every 3 months in those receiving aluminum-containing medications. (OPINION)
12.2.a In children with CKD prior to Stage 5, serum levels of aluminum should be measured yearly if children have been exposed to aluminum for 3 months or more in the prior year. (OPINION)
12.2.b Baseline levels of serum aluminum should be <20 µg/L. (OPINION)
12.2.c If levels of serum aluminum are between 20-60 µg/L, a search for and elimination of all sources of aluminum should be performed. (OPINION)
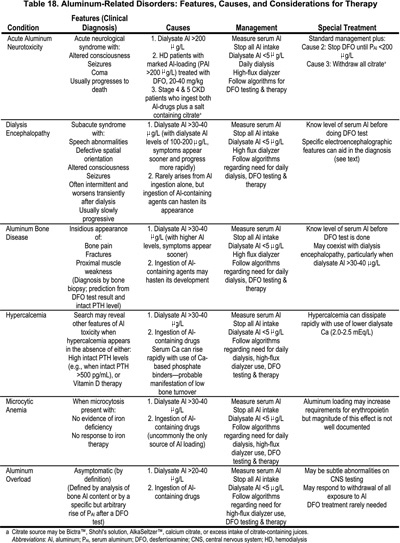
12.3 A deferoxamine (DFO) test should be performed if there are elevated serum aluminum levels (60-200 µg/L) or clinical signs and symptoms of aluminum toxicity (Table 18), or prior to parathyroidectomy if the patient has had aluminum exposure for at least 4 months or more. (OPINION) (See Algorithm 6 and Algorithm 7.)
12.3.a The test is performed by infusing 5 mg/kg of DFO during the last hour of the dialysis session with a serum aluminum measured both before DFO infusion and 2 days later, before the next dialysis session. (OPINION)
12.3.b The test is considered positive if the increment of serum aluminum is ≥50 µg/L. (OPINION)
12.3.c A DFO test should not be performed if the serum levels of aluminum are >200 µg/L to avoid DFO-induced neurotoxicity. (OPINION)
12.4 The presence of aluminum bone disease can be predicted by a rise in serum aluminum of ≥50 µg/L following DFO challenge combined with serum PTH levels of <150 pg/mL (16.5 pmol/L). (OPINION) However, the gold standard for the diagnosis of aluminum bone disease is a bone biopsy showing increased aluminum staining of the bone surface (>15%-25%) using an aluminum-specific stain, and often the presence of adynamic bone or osteomalacia. (EVIDENCE)
12.5 Asymptomatic patients receiving maintenance hemodialysis, with elevated levels of serum aluminum between 60-200 µg/L, should be treated with removal of aluminum based gels and intensive dialysis. Treatment with DFO is optional unless desired serum aluminum levels are not achieved. (OPINION)
Aluminum is widely present in nature, but most aluminum salts are quite insoluble. Moreover, only a tiny fraction of ingested aluminum is absorbed; this small amount is normally excreted by the kidney so that the body burden of aluminum does not increase. When there is a markedly reduced or absent kidney function, there is little or no ability to excrete aluminum and it can accumulate slowly. When aluminum is present in dialysate, it enters the body directly across the dialysis membrane, and the type of syndrome that develops depends on the rapidity and magnitude of aluminum accumulation. The various syndromes of aluminum toxicity were first identified in dialysis patients, but they can occur in both CKD Stage 4 patients and CKD Stage 5 patients not yet treated with dialysis. Because of their devastating nature and the difficulties in their management, it is essential that the clinical features of aluminum toxicity are recognized in addition to the specific biochemical methods used for their recognition. These problems have become substantially less common with the reduced use of aluminum gels as phosphate binders and proper purification of dialysate; however, aluminum toxicity still occurs. It is necessary to consider the means for proper monitoring and the appropriate diagnostic procedures needed to identify the various syndromes of aluminum toxicity.
Aluminum toxicity occurs in dialysis patients or CKD patients with GFR <30 mL/min/1.73 m2 (CKD Stages 4 and 5) because aluminum that is absorbed from the gut or that enters the body from dialysate or another parenteral route419 is not excreted, or is inadequately excreted by the diseased kidneys.420,421 When aluminum accumulates in dialysis patients, it is only slowly removed by dialysis because 90% of aluminum is bound to serum proteins (primarily transferrin422,423). The aluminum entering the body accumulates in various tissues, including bone, brain, parathyroid glands, and other organs.424,425 Such accumulation of aluminum can produce toxicity with several distinct syndromes, depending on the rate and magnitude of aluminum loading. The first to be described was dialysis encephalopathy (or dialysis dementia).426,427 Aluminum was then recognized as the cause of both “fracturing dialysis osteomalacia” (aluminum-related bone disease)427-429 and a microcytic anemia developing without iron deficiency.430-,432 A fulminant variant of dialysis encephalopathy, termed “acute aluminum neurotoxicity,” occurs with the sudden, marked elevation of serum aluminum levels and is commonly fatal.433,434 These disorders are briefly described below. The development and availability of a method to measure trace quantities of aluminum accurately in biological fluids and tissues435 permits detection of these disorders, and this methodology provides a means to identify patients with increased body burden of aluminum (see Algorithm 7).
Algorithm 6. Evaluation of Aluminum Neurotoxicity
Algorithm 7. Evaluation of Aluminum-Related Disorders: Considerations for DFO Test and Subsequent DFO Treatment
Acute aluminum neurotoxicity is diagnosed based on clinical features and the elevation of serum aluminum levels to 400-1,000 µg/L. It arises from aluminum contamination of dialysate, often to levels of 150-1,000 µg/L. As a rule, patients may become ill simultaneously in the same dialysis center. They develop agitation, confusion, myoclonic jerks, and major motor seizures; these symptoms are often followed by coma and death.433,434 The syndrome can also develop in patients with CKD Stages 3-4 (GFR <30 mL/min/1.73 m2) when they are given aluminum gels (to control hyperphosphatemia) plus sodium citrate (Bicitra ™ or Shohl's solution) for the correction of metabolic acidosis.436,437 Various citrate salts, including citric acid, sodium citrate, or calcium citrate, markedly enhance intestinal absorption of aluminum.203,438,439 Acute aluminum neurotoxicity can also appear in patients with large aluminum body load soon after the start of treatment with DFO in doses of 20-40 mg/kg.440,441 When acute aluminum neurotoxicity developed due to: a) very high dialysate aluminum levels; or b) the ingestion of both aluminum gels and citrate salts, most symptomatic patients had died.433,434,436,437 When the syndrome appeared in aluminum-loaded patients given DFO, some patients died; however, others survived when DFO treatment was stopped for several weeks and restarted later using a lower dose.440,441
Dialysis encephalopathy is an insidious disorder with symptoms generally appearing after patients have undergone dialysis for 12-24 months or even longer.426,427 Initial symptoms include subtle personality changes and a progressive speech disorder, characterized by stuttering, stammering, and hesitant speech, or even total inability to talk.442 Motor disturbances include twitching, myoclonic jerks, and motor apraxia. Auditory and visual hallucinations, spatial disorientation, and paranoid behavior are common. These features can fluctuate widely and are characteristically worse shortly after dialysis. With time, the symptoms become persistent and worsen, seizures appear, and most untreated patients have died within 6-12 months after the onset of symptoms.426 The only distinctive laboratory findings were substantial elevations of serum aluminum, usually 150-350 µg/L. Findings from an electroencephalogram (EEG) differ from the generalized slowing noted with other causes of metabolic encephalopathy. The diagnosis of these neurological disorders rests on clinical suspicion, the finding of elevated serum aluminum levels, and the EEG features. New cases of this syndrome disappeared after the initiation of water purification using reverse osmosis.
Aluminum-related bone disease was first described in certain specific geographic areas of the U.K. and the U.S.428,443; there was a suspicion of aluminum toxicity because many patients developed clinical features of dialysis encephalopathy.428,443 Epidemiological studies showed that this disorder—which presented with bone pain, a characteristic “waddling” gait, proximal muscle weakness, and fractures 430 —was associated with dialysate aluminum levels >100 µg/L.429 The disorder was limited to certain geographical regions, and aluminum-contaminated dialysate was considered the only source of aluminum loading. Later, sporadic cases appeared in dialysis centers where elevated dialysate aluminum levels were never found,444,445 and it was shown that small quantities of aluminum are absorbed from ingested aluminum gels.446 Such sporadic cases of aluminum bone disease have become less common since the use of aluminum gels was stopped or their dosage reduced substantially.327,447
Patients with aluminum-related bone disease often exhibit hypercalcemia,448,449 and PTH levels which are variably elevated, particularly with older C-terminal or mid-region 1st PTH-IMA assays.449,450 Some of these patients had radiographic features of subperiosteal erosions and, when parathyroidectomy was done, the clinical features worsened. Bone biopsies revealed typical aluminum-related bone disease, and the term pseudohyperparathyroidism was applied to such patients.450 Other observations have documented the appearance or worsening of skeletal symptoms when patients with aluminum-related bone disease or aluminum loading had their PTH levels reduced by either parathyroid surgery451 or by treatment with an active vitamin D sterol.367,452
Indirect methods to identify aluminum-related bone disease were sought. Serum aluminum levels were elevated in afflicted patients, with values usually >100 µg/L; however, similar levels were found in many patients lacking bone biopsy evidence of aluminum-related bone disease.327,453 The DFO infusion test, using DFO in doses of 20-40 mg/kg, was introduced to identify those with aluminum bone disease.454,455 The results indicated that the rise in aluminum correlated better with the total bone aluminum content than with surface staining of aluminum.456,457 Further, the presence of bone surface staining for aluminum of >15%-25% showed a close association with clinical symptoms and with bone biopsy features of reduced bone formation and even osteomalacia, the histological features of aluminum bone disease.458-460
Population studies suggested that the combination of the increment of serum aluminum after DFO combined with PTH levels <150 pg/mL (16.5 pmol/L) provided better sensitivity and specificity to predict aluminum bone disease than the DFO test alone.455,461 Also, it was found that the sensitivity of the DFO test was reduced substantially in patients with no known exposure to aluminum for 6 months or longer.461 Most information indicates that serum aluminum levels only reflect recent aluminum intake.462
Problems arose with use of the DFO test. Isolated reports documented permanent visual loss from ophthalmological damage after one DFO test with a dose of 40 mg/kg.463,464 Furthermore, the use of DFO, 20-40 mg/kg, was associated with fulminant and fatal mucormycosis in an unacceptable number of dialysis patients.465 As a consequence, there has been reluctance to use a DFO test dose of 40 mg/kg, and smaller doses have been evaluated.466-468
Prevention of aluminum toxicity is preferable to use of toxic methods for treatment, particularly with the mortality of the neurological disorders and high morbidity of the bone disease. Periodic monitoring of serum aluminum levels and assessment of aluminum in dialysate are essential for its prevention.
The evidence for the devastating neurological and skeletal disorders produced by contamination of dialysate with aluminum is compelling. However, these reports are not prospective, randomized trials, and such trials can never be done.
Serum Aluminum Levels and Frequency of Monitoring
Early studies of serum aluminum measurements in dialysis patients indicated that serum aluminum levels reflect relatively recent exposure to aluminum.424,469 The population studies based on a single measurement of serum aluminum provide no information on the optimal frequency to monitor serum aluminum levels. The purpose of monitoring serum aluminum levels is: (a) to identify excessive aluminum intake or absorption in individual patients; or (b) to aid in recognition of accidental contamination of dialysate with aluminum. The recent reported accidental events with aluminum contamination of dialysate were often detected because neurological symptoms appeared in dialysis patients434,470,471; deaths often occurred before the source was identified or corrected. Under these circumstances, dialysate aluminum levels were markedly elevated (>200 µg/L). Although the dialysate aluminum levels were high, dialysate monitoring may not be frequent enough to detect a problem, as water aluminum levels can vary from day to day. Twice-yearly monitoring of serum aluminum would be capable of detecting the slow accumulation of aluminum from oral absorption or from “modest” dialysate contamination (dialysate aluminum levels of 20-40 µg/L). Indirect evidence can be derived from studies showing the increment of serum aluminum levels during the ingestion of aluminum gels or from studies of serum aluminum levels after the withdrawal of aluminum gels. These studies suggest that serum levels change very slightly over 2-3 weeks of ingesting aluminum gels (when there is no intake of citrate). A prospective, controlled study in children and young adults undergoing peritoneal dialysis166 with measurements of aluminum levels every 2 months showed a slow increase of basal (or unstimulated) serum aluminum from 22.4 ± 30 µg/L to 57.8 ± 13 µg/L after 12 months with intake of aluminum hydroxide, 30 mg/kg BW, a dose considered “safe” in children with CKD165; this contrasts to serum aluminum decreasing from 21.6 ± 2.3 to 13.2 ± 1.3 µg/L in the group given only calcium carbonate.166 In the aluminum-gel group, serum aluminum levels had increased significantly by 4 months (P < 0.05), and the levels differed from the group not ingesting aluminum gels (P < 0.05). These studies showed that “safe” and “low” aluminum hydroxide doses failed to prevent significant rises in serum PTH and alkaline phosphatase, and worsening of hyperparathyroid bone disease on repeat bone biopsy after 13 months. Such data suggest that measuring serum aluminum every 4 months would be capable of detecting increased aluminum burden from oral aluminum gels.
The changes in serum aluminum after withdrawal of aluminum gels provides information on how rapidly serum aluminum levels fall after they were known to be elevated. In 32 hemodialysis patients, serum aluminum fell from 105 ± 21 µg/L to 34 ± 11 µg/L, 8 months after aluminum gels were stopped; the fall was slow with the magnitude of reduction being –67.3 ± 5.1% of “baseline” after 8 months.244 In another study of individual serum aluminum values measured every 6 months in 13 patients,472 serum aluminum levels—ranging up to 66 µg/L while the patients received aluminum gels—fell below 20 µg/L at 6 months in all except one patient who “consumed large doses of Al(OH)3.”
Ingestion of Aluminum Gels and Aluminum Toxicity
Is there a dose of aluminum gels that is effective and yet safe for long-term use? The safety of aluminum gels cannot be evaluated unless there is confidence that the dialysate contains no aluminum. The prevalence of aluminum-related bone disease has decreased markedly over the last 10-15 years in association with increased use of nonaluminum phosphate-binding agents in combination with purification of water used for dialysate.327,447A large population study of 289 patients reported that the cumulative dose of aluminum is a continuous variable predicting the risk of aluminum bone disease compared to other bone pathology, based on a difference of total intake of 1 kg of aluminum hydroxide (equal to two Alucap ™ capsules thrice daily for 1 year).473 One study of 17 patients with bone evaluated postmortem showed a close correlation (r = 0.80) between bone aluminum content and the cumulative intake of aluminum gels.474 Another report of 92 dialysis patients undergoing bone biopsy also showed a close relationship between bone aluminum content and total intake of Al(OH)3 (r = 0.83)475; moreover, bone aluminum levels were trivially elevated above normal in the dialysis patients who never ingested aluminum gels. In these reports,474,475 the finding of aluminum bone disease was limited to patients with the greatest cumulative dose of aluminum gels; the latter is related to the duration of dialysis treatment. Among 253 Italian hemodialysis patients ingesting aluminum hydroxide, there was a relatively close association between serum aluminum levels and bone aluminum content; 93% of patients with serum aluminum levels >60 µg/L had bone aluminum content >60 mg/kg BW.476 A study in children and young adults on CAPD166 showed evidence of aluminum accumulation based on the result of a DFO infusion test (using 40 mg/kg BW), after only 1 year of consuming a “low dose” of aluminum gels; thus, the increment of serum aluminum rose from 58 ± 65 µg/L to 206 ± 153 µg/L. These data point to the risk of ingestion of aluminum gels for any length of time. If aluminum gels are ingested, care must be taken to avoid the concomitant intake of any compound containing citrate because of the profound effect of citrate to enhance aluminum absorption.438 Such intake is difficult to monitor since several over-the-counter preparations contain citrate (e.g., AlkaSeltzer ™ or Citracal ™); they can be consumed without any knowledge of those treating the patient.477
Monitoring Serum Aluminum and Recognition of Aluminum Toxicity
One study reported monitoring serum aluminum twice yearly over a 4-year period, 1984-1987.472 There were 1,193 Belgian dialysis patients in dialysis units with “negligible aluminum contamination of dialysate”; from 1986 onward, water aluminum concentrations were constantly <3 µg/L. Data analysis involved individual measurements of serum aluminum rather than mean values for each patient. In a subset of 77 patients with bone biopsies, 31% demonstrated aluminum bone disease. With a cut-off serum aluminum of 60 µg/L, there was a sensitivity and specificity for detecting aluminum bone disease of 82% and 86%, respectively. Among the total group of patients, six were diagnosed with dialysis encephalopathy, based on clinical features and EEG abnormalities. The median serum aluminum was 121 µg/L (range, 15-462 µg/L) in patients with dialysis encephalopathy compared to 42 µg/L (range 4-140 µg/L) in matched controls. Most patients had undergone dialysis for some time before these aluminum measurements were initiated.
DFO Infusion Test as a Predictor of Aluminum Bone Disease
Because of side-effects with the DFO test using doses of 20-40 mg/kg BW,463,464,478 such doses have been abandoned in favor of lower ones.466-468 In a study of patients from European countries, North Africa, and South America, DFO tests, both 10 mg/kg BW and 5 mg/kg BW, were given to 77 hemodialysis patients with bone biopsies. Both doses were given to 71 patients, with alternate order of giving the two doses. The indications for bone biopsy included a serum aluminum level above 60 µg/L or in those with serum aluminum below 60 µg/L, the presence of symptoms of osteodystrophy, radiological signs of osteodystrophy, or the need for calcitriol therapy or parathyroidectomy based on biochemical parameters. Based on a chemical aluminum content of bone >15 µg/g wet weight combined with positive aluminum staining (>0%), 57 patients were classified as having aluminum overload; 15 others were classified with aluminum bone disease based on aluminum staining >15 % of bone surface and BFR reduced below normal. Using the DFO dose of 5 mg/kg BW, the combination of iPTH <150 pg/mL (150 ng/L) and an increment of serum aluminum >50 µg/L had a sensitivity of 87% and a specificity of 95% for detecting aluminum bone disease. Use of the 10 mg/kg DFO dose provided no additional benefit.
Several studies evaluated low doses of DFO but did not compare the results to findings on bone biopsy. The increment of serum aluminum was evaluated after two DFO tests, 30 mg/kg, and a total dose of 500 mg, in 22 hemodialysis patients; the lower dose was as efficacious in detecting evidence of aluminum overload as the higher dose.479 Other reports utilized still lower doses of DFO: doses of 0.5, 2.5, and 5.0 mg/kg were each given to five patients with serum aluminum levels above 40 µg/L and the change in total and ultrafilterable aluminum measured after 44 hours.467 The total and ultrafilterable aluminum rose with each dose, suggesting a reliable test value of even the lowest dose. Another study described repeated use of doses of 0.5 mg/kg, demonstrating significant chelation of aluminum with this so-called minidose.468
The evidence that aluminum is absorbed from aluminum hydroxide and other aluminum-containing compounds is indirect; however, the methodology for measuring true aluminum absorption using a stable isotope and mass spectroscopy is very expensive, has limited availability, and is likely to be done in very small numbers of patients. The close relationship between the cumulative aluminum intake and the skeletal accumulation of aluminum, along with the reduced prevalence of aluminum bone disease as the use of aluminum gels has decreased, provides only indirect—but convincing—evidence to recommend that aluminum gels not be used as phosphate binders, except for a very short periods of time.
The substantial reduction in prevalence of aluminum bone disease, and the apparent disappearance of this problem in dialysis units where aluminum gels are not used as phosphate binders, makes this a problem that may be disappearing.
Prospective comparison of aluminum gels and calcium-based phosphate binders was done in a small numbers of patients and was limited to a year of therapy.166 Also, the studies that showed the close correlation between the quantity of aluminum ingested and that present in bone at postmortem474 or on biopsy476 were not prospective studies.
Awareness of the various manifestations of aluminum toxicity by all health-care providers will allow early recognition of aluminum loading and aluminum toxicity in CKD patients. This will permit the earlier diagnosis and treatment of the syndromes of aluminum toxicity, thereby leading to reduced morbidity and disability. Use of the recommended low dose for the DFO test will minimize any risk of side-effects from the test. Such better safety should lead clinicians to use the DFO test with more confidence in clinical conditions when it may be useful or necessary. Through proper monitoring of serum aluminum levels and the interpretation of these values, there will be earlier recognition of aluminum loading, with a greater ability to prevent the occurrence of aluminum toxicity.
Longitudinal studies with the measurement of serum aluminum at every 6 months from the very outset of dialysis, combined with a subsequent DFO test and bone biopsy in randomly selected patients and others chosen because serum aluminum levels rise >40 µg/L, could provide information on the “peak” aluminum levels at which there may be a risk of aluminum loading or the development of aluminum bone disease.
Limited long-term trials with very low doses of aluminum gels, which remain the most “potent” of phosphate binders, would be useful. Such doses, however, almost certainly would need to be combined with another type of phosphate-binding agent.
Large, prospective, long-term trials with the use of “low doses” of aluminum gels as phosphate binders would be useful. Those who remain convinced that low doses of aluminum are safe (and there remain some with this viewpoint) should seem compelled to design such trials to prove the point. Whether low doses of aluminum gels might be effective and safe when they are given in combination with continued “minidoses” of DFO treatment468 would be useful to consider for a prospective trial, particularly with the growing concern about potential risks of calcium-based phosphate binders.