4.1 In CKD patients (Stages 1-4), the serum level of phosphorus should be maintained at or above the age-appropriate lower limits (EVIDENCE) and no higher than the age-appropriate upper limits. (OPINION)
4.2 For children with kidney failure (CKD Stage 5), including those treated with hemodialysis or peritoneal dialysis, the serum levels of phosphorus should be maintained between 3.5-5.5 mg/dL (1.13-1.78 mmol/L) during adolescence and between 4-6 mg/dL for children between the ages of 1-12 years. (EVIDENCE)
4.3 In children with renal tubular phosphate wasting, or other causes of hypophosphatemia, hypophosphatemia should be corrected via dietary modification, enteral supplementation, or reduction in the use of phosphate binders. (EVIDENCE)
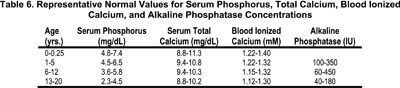
There are substantial effects of age on the fasting serum concentration of phosphorus. Serum phosphorus levels in infants range from 4.8-7.4 mg/dL (mean 6.2 mg/dL) in the first 3 months of life, and decrease to 4.5-5.8 mg/dL (mean 5.0 mg/dL) at age 1-2 years.70 The higher serum phosphorus concentration in infants is attributed to an increased fractional phosphate reabsorption, possibly further augmented by a low GFR.70 In mid-childhood, values range from 3.5-5.5 mg/dL (mean 4.4 mg/dL) and decrease to adult values by late adolescence.71-73 Representative target ranges for serum phosphorus concentration in children with CKD are depicted in Table 6. The normal range for serum phosphorus concentration can vary somewhat between laboratories.
In healthy children and in children with GFR ranging from 25-50 mL/min/1.73 m2 (approximately Stage 3 CKD), the serum phosphorus concentration decreased after breakfast to a nadir in late morning, and increased during the early afternoon74, exhibiting a circadian pattern similar to that observed in healthy adult subjects ingesting typical diets.75 The amplitude of the circadian variation in serum phosphorus concentration in healthy adolescents (3.0 mg/dL) is greater than that in healthy adults (1.2 mg/dL)76. Restriction of dietary phosphorus induces substantial decreases in serum phosphorus levels during late morning, afternoon, and evening, but little or no change in morning fasting phosphorus levels.75 Thus, values obtained in the afternoon are more likely to be affected by diet and may be more useful in monitoring the effect of phosphorus restriction or administration of phosphate-binding agents on serum phosphorus concentrations.
Hyperphosphatemia leads to 2° HPT and elevated blood levels of PTH by: (a) lowering the levels of ionized calcium; (b) inhibiting the production of 1,25(OH)2 D3; and (c) directly stimulating PTH secretion.77,78
These processes lead to high-turnover bone disease and other adverse consequences of excess PTH, in part due to an increase in calcium-phosphate product (CaXP)79-82 and in adults, is associated with increased morbidity83,84 and mortality.85-88 With respect to vascular calcification, hyperphosphatemia exerts a direct calcifying effect on vascular smooth muscle cells.89 Calcification of coronary arteries, cardiac valves, and pulmonary tissues results in cardiac disease, the leading cause of death in patients with CKD.83,90-92 In young adults who developed CKD in childhood years, there is high incidence of coronary artery calcification, associated with an elevated CaXP.80,93 It is therefore imperative to prevent hyperphosphatemia and maintain serum phosphorus levels within the normal range.
Children with CKD may have renal tubular wasting of phosphorus. Fanconi syndrome due to cystinosis is the most common etiology in childhood. A variety of other genetic disorders and drug or toxin exposure may also cause Fanconi syndrome. In addition, some children develop CKD and hypophosphatemia due to Dent's disease. Hypophosphatemia may also be secondary to excessive use of phosphate binders, vitamin D deficiency, inadequate dietary intake of phosphorus intake, or other tubular disorders. Chronic hypophosphatemia in children results in rickets and poor growth.
Among the factors that contribute to 2° HPT in CKD patients are phosphate retention and/or elevated levels of serum phosphorus. In adult CKD patients, hyperphosphatemia is associated with increased morbidity and mortality.79-86 Conversely, in adults, hypophosphatemia is associated with increased mortality. In children with CKD, hypophosphatemia leads to rickets and growth retardation. Therefore, the maintenance of normal serum levels of phosphorus in CKD patients is critical for the prevention of abnormalities in PTH metabolism, and for the reduction of morbidity and mortality.
While available experimental data support a direct role of phosphorus in the regulation of PTH secretion,77,78 the data in humans are less straightforward. One study has shown elevated PTH levels in patients with serum phosphorus levels >6.2 mg/dL (2.0 mmol/L).83 On the other hand, other studies have failed to demonstrate consistent changes in PTH levels across a range of serum phosphorus levels,94 and no direct correlation between the level of serum phosphorus and PTH has been established.78 Many studies measuring serum PTH levels are confounded by the use of phosphate binders and vitamin D, thus precluding the evaluation of a direct association between serum phosphorus and PTH levels. Based on available evidence and upon clinical experience it is the opinion of the Work Group that, for adults and for children with CKD, elevated phosphorus levels in CKD and dialysis patients contribute to the development of 2° HPT.
In order to eliminate the potentially confounding influence of aluminum-containing phosphate binders on outcomes, only studies of adult dialysis patients, and only those published after 1990, were included in the data analysis. Four studies meet these criteria, and all are observational or cross-sectional in design.83,85-87 These studies correlate serum phosphorus levels with multiple end-points in patients treated with hemodialysis.
The four cross-sectional studies83,85-87 that met the inclusion criteria evaluated the association of serum phosphorus levels with extraskeletal outcomes. Two studies evaluated the relative risk of mortality associated with serum phosphorus levels in patients treated with hemodialysis. In one study, a reference serum phosphorus range of 4.6-5.5 mg/dL (1.49-1.78 mmol/L) was used85; the relative risk of mortality increased with serum phosphorus levels >6.5 mg/dL (2.10 mmol/L). In the other study, a reference range of 5-7 mg/dL (1.61-2.26 mmol/L) was used86; the relative risk of mortality increased with serum phosphorus levels less than or greater than this range. The increase in mortality was particularly significant for levels of phosphorus >7 mg/dL (2.26 mmol/L) or <3 mg/dL (0.97 mmol/L). Serum phosphorus levels >2.5 mg/dL (0.81 mmol/L) may be associated with abnormalities in bone mineralization such as osteomalacia.94
In another study, serum phosphorus levels >6.2 mg/dL (2.00 mmol/L) were associated with increased blood pressure, hyperkinetic circulation, increased cardiac work, and high arterial tensile stress.83 One study failed to find an association between serum phosphorus levels and quality of life.94
In patients with tubulopathy and hypophosphatemia, phosphate therapy is a critical component of the treatment of the bone disease, as is provision of alkali and vitamin D.
The available evidence supports an association between serum phosphorus levels both above and below the normal range with poor outcomes, including mortality.
In adults with CKD, cross-sectional studies have established a correlation between serum phosphorus levels and various extraskeletal outcomes, but this correlation does not rise to the level of causality. Further, the studies of higher methodological quality85,86 relied on data from 1990 or earlier, indicating that their results may have been confounded by the use of aluminum hydroxide and/or by less-aggressive vitamin D therapy. To date, studies performed in dialysis patients have failed to conclusively demonstrate a reduction in morbidity or mortality, through dietary intervention or the use of phosphate binders to lower serum phosphorus levels to the suggested target range.
In children with CKD, there are no prospective studies or retrospective analysis to establish the effect of normalization of serum phosphorus on clinical outcome outside of rachitic bone disease.95 Even the successful treatment of rickets generally requires additional pharmacological therapy, making the independent impact of normalization of serum phosphorus uncertain.
This Guideline supports intensive control of serum phosphorus in patients with CKD. Most data indicate that <30% of dialysis patients are able to maintain phosphorus in the suggested target range. The goal should be to increase the percentage of patients in this target range. Successful implementation will require an increased dietitian-to-patient ratio, educational tools to increase patient compliance, as well as studies to further explore the feasibility of dialytic techniques that are better able to control serum phosphorus levels (such as nocturnal or daily hemodialysis), and the widespread availability and affordability of different phosphate binders, regardless of patient insurance status.
Longitudinal studies of patients with CKD are needed, evaluating the effects of controlling serum phosphorus in the target range on morbidity and mortality.