GROWTH AND DEVELOPMENT OF THE SKELETON
During childhood and adolescence, total skeletal calcium increases from approximately 25 g at birth to 900 g and 1,200 g in adult females and males, respectively. Attainment of optimal peak bone mass by young adulthood is thought to be the best protection against osteoporosis later in life. 1 Therefore, childhood and adolescence are particularly critical periods for the establishment of life-long bone health. While peak bone mass is strongly influenced by genetic factors, full genetic potential is attained only if nutrition, growth, physical activity, and metabolic and endocrine function are optimal in children. The clinical features of renal bone disease unique to childhood relate to distinctions between the growing and the fully-grown skeleton. The metabolic process of skeletal modeling throughout growth dictates that pediatric-specific recommendations for the management of the bone disease of CKD be developed.
BONE FORMATION
Formation of the skeleton occurs by two processes of ossification—intramembranous and endochondral. Intramembranous ossification is the direct mineralization of vascular connective tissue membrane in the plate-like bones of the skull, facial bones, mandible, and clavicle. The transformation of mesenchymal cells into osteoblasts and production of osteoid matrix convert the primitive mesenchyme into bone. In contrast, bones that involve joints and bear weight form by endochondral ossification. Endochondral bone formation is the result of ossification of an intermediate cartilage model that is derived from mesenchyme and represents the position and shape of the bone to be formed at that site. This provides a mechanism for the formation of bone during growth. In the long bones of the extremities, the primary center of ossification is located in the central portion of the cartilage model. Proliferation and hypertrophy of chondrocytes and elaboration of matrix result in linear growth. Ossification proceeds toward the end of the bone and ultimately forms the growth plate (epiphyseal plate or physis) that is the predominant site of longitudinal bone growth. With continued maturation, the growth plate thins and eventually disappears with fusion of the epiphyseal and diaphyseal ossification centers. Epiphyseal union occurs at an earlier age in females than males. Knowledge about the appearance of various ossification centers in the carpal bones is used to determine a child's maturational age, or “bone age.”
BONE MODELING
The shape and structure of bones are continuously modified and renovated by two different processes during growth: modeling and remodeling. Both processes result in the replacement of old bone tissue with new bone. The remodeling cycle of bone resorption and formation takes place throughout life and is vital for microdamage repair and maintenance of skeletal integrity. In contrast, modeling predominates during growth and promotes formation of new bone at locations different from the sites of bone resorption. This results in increased bone mass and modification of bone shape. For example, increases in cortical bone diameter of the diaphysis are due to concurrent bone formation on the periosteal (outer) surface and bone resorption on the endosteal (inner) surface. In contrast, as the bone grows in length, the wide metaphyseal region is converted to a narrow diaphysis through resorption of the periosteal surface and bone formation on the endosteal surface. Finally, long bones drift in a lateral direction during growth due to relatively greater resorption along the medial edge of the bone and formation along the lateral edge.
In conclusion, bone growth during childhood and adolescence involves the complex coordination of varied cell activities on specific bone surfaces. Cartilage proliferation, bone modeling, and epiphyseal closure are under the direct influence of a variety of hormones and growth factors, such as growth hormone (GH), thyroid hormone, estrogen, testosterone, parathyroid hormone (PTH), vitamin D, and insulin-like growth factors (IGF).2 Each of these factors may be disordered in CKD, with important effects on bone structure and maturation.
CLINICAL MANIFESTATIONS OF BONE DISEASE IN CHILDREN WITH CKD
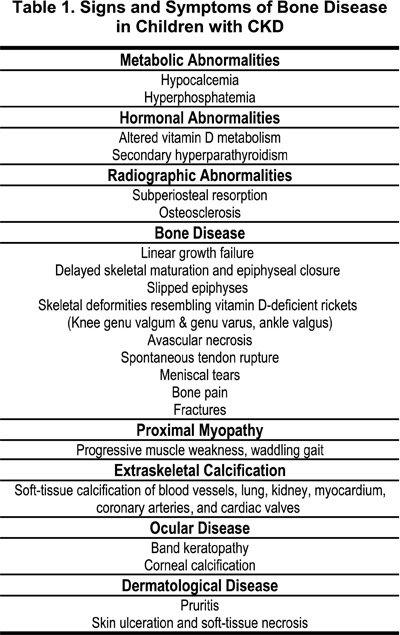
Renal osteodystrophy is an early, universal, and pervasive consequence of CKD that may develop prior to any clinical manifestations of renal failure. The clinical manifestations of renal bone disease seen in adults may also appear in childhood. However, the effects of abnormal bone and mineral metabolism on endochondral ossification during growth result in complications in the epiphyseal region that are unique to the children with CKD. The clinical signs and symptoms of bone disease in children with CKD are summarized in Table 1, highlighting those seen exclusively during growth and development.
GROWTH RETARDATION AND SKELETAL MATURATION DELAY
Growth retardation is a frequent feature of CKD in children. Skeletal maturation is usually delayed commensurate with the growth deficit. In the 2002 North American Pediatric Renal Transplant Cooperative Study Annual Report, growth status is described in over 5,000 children and adolescents with CKD Stages 2-4. Use of age- and gender-specific standard deviation (SD) scores revealed that more than one-third of patients had a height SD score of less than −1.88 (3rd percentile). Height deficits were observed across all ages, from infants through adolescents; however, the SD score deficits were greatest among the children with early-onset and long-standing CKD. Nevertheless, significant height deficits were also observed in children with mild to moderate CKD. Among children with an estimated glomerular filtration rate (GFR) of 50-75 mL/min/1.73 m2 (Stages 2-3), 22% had a height SD score of less than −1.88; among children with an estimated GFR of 25-50 mL/min/1.73 m2 (Stages 3-4), 38% had a height SD score of less than −1.88.
The growth retardation and delayed maturation in children with CKD is multifactorial. Potential contributing factors include prior steroid therapy, chronic metabolic acidosis, anorexia, inadequate nutrient (vitamins, trace minerals) and caloric intake, hyposthenuria and sodium depletion, inadequate insulin-like growth factor I (IGF-I) availability due to increased serum levels of IGF-binding protein 3 (IGFBP-3), inadequate testosterone and estrogen production during puberty, and bone disease, including severe rachitic-like lesions. The contribution of renal bone disease to growth failure is unclear; however, treatment with 25(OH) vitamin D3 therapy in children with CKD resulted in improved growth.3
MUSCULOSKELETAL DEFORMITIES
Clinical manifestations of the bone disease associated with CKD in children generally involve the musculoskeletal system. Nonspecific symptoms such as bone pain, muscle cramps with repetitive motion activities, and a decrease in normal muscle functions used in activities of daily living may be seen in children with CKD. Fractures may occur in children with CKD with deformed bones subjected to the trauma of normal childhood activities. The evaluation and treatment of orthopedic complications are discussed in greater detail in Guideline 3.
During childhood and adolescence, bony deformities may develop, most commonly involving rapidly growing bones of the extremities. Vitamin D deficiency in CKD results in skeletal deformities resembling vitamin D-deficient rickets, such as a rachitic rosary, widening of the metaphysis, frontal bossing, craniotabes, ulnar deviation, and pes varus. Histologically, there is disorganization in the growth plate and subjacent metaphysis. The hypertrophic zone of the growth plate demonstrates an increase in cell number and loss of the normal columnar cell pattern. The disordered cartilage is resorbed and no longer provides adequate scaffolding for bone deposition. The subjacent metaphyseal fibrosis may interfere with vascularization and maturation of the growth plate.
The growth plate in children with CKD is vulnerable to injury; the hypertrophic zone is most vulnerable to shearing injuries. Morphological studies on the epiphyses have demonstrated a dense fibrous tissue that disrupts the connection between the epiphyseal plate and the metaphysis.4 These abnormalities, along with hyperparathyroid erosions of bone, result in an increased risk for slipped epiphyses (physiolysis) and genu valgum. Possible sequelae of slipped epiphyses include severe varus deformity, osteonecrosis, chondrolysis and degenerative joint disease. The orthopedic approach to correction of extremity deformities in children with CKD often requires an osteotomy. It is clinically recognized that healing of the bone after such a procedure proceeds poorly in the face of severe secondary hyperparathyroidism (2° HPT). Therefore, it is urged that biochemical control of the hyperparathyroidism be accomplished prior to performance of the osteotomy to enhance success of the procedure.
BONE MINERAL ACCRETION AND PEAK BONE MASS
The impact of CKD on peak bone mass is not known. However, increased bone resorption on the periosteal and endosteal surfaces due to 2° HPT may compromise bone microarchitecture, density, and dimensions. Biopsy studies in adults with kidney disease have demonstrated a 40% reduction in cortical bone mass 5; therefore, it is likely that CKD during childhood compromises bone mineral accretion and results in inadequate peak bone mass.
As outlined in these guidelines, the management of bone disease in children with CKD requires careful monitoring for skeletal complications in the growing child, and strategies to optimize bone mineral accretion. In addition, dual-energy X-ray absorptiometry (DXA) is subject to several limitations that limit its usefulness in children. These include the inadequacy of pediatric reference data across varied maturational stages, ethnic groups, and gender groups in healthy children, and difficulties in the interpretation of DXA results in children with impaired growth, altered body composition, or delayed maturation due to childhood illness.
OVERVIEW OF PRIOR PEDIATRIC KDOQI GUIDELINES: IMPACT OF RENAL FUNCTION, GROWTH HORMONE, AND NUTRITION ON THE MANAGEMENT OF BONE DISEASE IN CHILDREN WITH CKD
Prior KDOQI Pediatric Work Groups have published recommendations regarding the assessment of renal function in children with CKD, the indications and monitoring of growth hormone therapy, and the unique nutritional needs of these children. These guidelines impact the management of bone disease in CKD and are summarized here.
Estimation of GFR in Children with CKD
Among children, the Schwartz and Counahan-Barratt formulae provide clinically useful estimates of GFR.6,7
Growth and Nutrition in Children on Dialysis
Scheduled, interval measurements of growth and nutrition parameters should be obtained to provide optimal care of the nutritional needs of children on dialysis.
Supplemental nutrition support should be considered when a patient is not growing normally (i.e., does not have a normal height velocity) or fails to consume the Recommended Dietary Allowances (RDA) for protein and/or energy. Supplementation for the oral route is preferred followed by enteral tube feeding.
Recommendations for the Use of Recombinant Human Growth Hormone (rhGH) for Children Treated with Maintenance Dialysis
Treatment with rhGH in children with CKD should be considered under the following conditions:
Children who have (a) a height for chronological age more negative than 2.0 SD; or (b) a height velocity for chronological age SD more negative than 2.0 SD; (c) growth potential documented by open epiphysis; and (d) no other contraindications for GH use.
Prior to the consideration of the use of rhGH, there should be correction of (a) insufficient intake of energy, protein and other nutrients; (b) acidosis; (c) hyperphosphatemia (the level of serum phosphorus should be less than 1.5X the upper limit for age); and (d) 2° HPT.