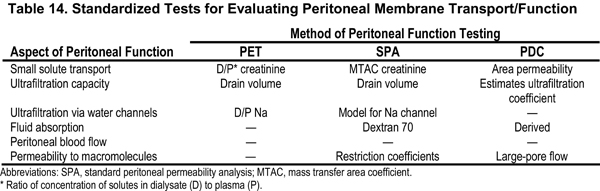
Total solute clearance and peritoneal effluent volume ultimately are influenced by peritoneal membrane transport characteristics. Multiple tests are documented to be efficacious for determining peritoneal membrane transport. None of these tests has been shown to be clinically superior to the others (see Table 14).
3.1 Each center should choose one of these tests to use when characterizing peritoneal transport in their patients.
3.2 Baseline peritoneal membrane transport characteristics should be established after initiating a daily PD therapy.
3.3 Data suggest that it would be best to wait 4 to 8 weeks after starting dialysis to obtain this baseline measurement.
3.4 Peritoneal membrane transport testing should be repeated when clinically indicated (see Table 15).
3.5 All measurements of peritoneal transport characteristics should be obtained when the patient is clinically stable and at least 1 month after resolution of an episode of peritonitis.
After PD therapy is initiated, total solute removal is related to residual kidney and peritoneal effluent volumes and the concentration of the solute in question in each of those volumes. The background for, definitions of, and frequency of how and what to measure to determine total solute removal or clearance are outlined in CPG 2 and CPR 2. During a typical PD dwell, peritoneal effluent drain volume and concentration of solutes in that drain volume will vary from patient to patient and are dependent on the individual patient's peritoneal membrane transport characteristics, infused volume/exchange, concentration and type of osmotic agent used, rates of lymphatic absorption of fluid, and dwell time/exchange.211 Although in our goal to replace lost RKF, we have been focused on the movement of solutes and fluid from blood to the peritoneal cavity (and ultimately, by draining the peritoneal fluid, removal from the body), it is important to note that solutes (ie, osmotic agents) and fluid also potentially are absorbed from the peritoneal cavity. To most efficiently optimize solute and fluid removal in each patient, one must know and understand each individual's peritoneal membrane transport characteristics and recognize that there is potential that they may change over time.212,213
Definitions
Two of the typical laboratory measurements routinely obtained in PD patients are: (1) those used to quantify and document amount of solute removed from the body (such as the weekly Kt/Vurea or CCr described previously), and (2) tests that classify peritoneal membrane transport characteristics (described next). Tests that measure peritoneal membrane transport characteristics are designed to define or classify an individual patient's rate of solute diffusion and potential fluid removal, not quantify actual amount of solute or volume of fluid removed. After an individual patient's peritoneal membrane transport characteristics are defined, one can use such data to guide prescription management and predict what the delivered solute removal may be with a certain prescription. As noted, it is recommended that dialysate and urine be collected and solute removal be measured to accurately quantify a patient's delivered dose of dialysis.
Each center should choose one of these tests to use when characterizing peritoneal transport in their patients.
It is known that peritoneal membrane transport characteristics vary from patient to patient. To optimize solute removal and ultrafiltration volumes, it is helpful to know each patient's individual peritoneal membrane transport properties. Multiple tests have been developed to evaluate various aspects of peritoneal membrane function (see Table 14). There have been no prospective randomized trials designed to determine which test is best for prescription management. Each test has its strengths and weaknesses, and all are useful. These have been reviewed recently.214
Traditionally, peritoneal membrane transport/function has been assessed by using the standard PET.211 The PET has been standardized both procedurally and interpretably to classify peritoneal membrane function. It was designed and initially used primarily to evaluate small-solute transport characteristics, and although ultrafiltration properties of the peritoneal membrane are linked, the original PET was not designed to differentiate all the variations in peritoneal membrane transport/function that result in pathological alterations in ultrafiltration capacity.
A modification of the original PET using 1.36%/1.5% dextrose/dextran 70, called the standard peritoneal permeability analysis (SPA), was developed to better evaluate mass transfer area coefficients (MTAC) of small- and middle-molecular-weight solutes and also better determine residual volume and ultrafiltration kinetics.215 The 1.36%/1.5% dextrose/dextran 70 solutions were chosen so there would be less of an osmotic gradient for ultrafiltration and therefore one would be able to better determine the true diffusive MTAC characteristics of the membrane in a situation in which there would be less ultrafiltration and its associated convective removal of solutes.
The standard PET subsequently was modified, and 3.86%/4.25% dextrose solutions were substituted to maximize crystalloid osmotic ultrafiltration and optimize the ability to evaluate pathological variations in ultrafiltration capacity.216 This modification allows one to evaluate aquaporin-mediated water transport and the sodium- versus water-removal characteristics of peritoneal transport. The PET and SPA use single dwells and direct measurements to characterize peritoneal transport properties.
Another procedure, the PDC test, uses data from multiple dwells (typically 5) performed during a 24-hour period.217 Data are combined in a mathematical model to estimate peritoneal transport characteristics. In addition to establishing MTAC, the PDC test is better able to determine peritoneal fluid absorption rates and macromolecule permeability.
There are geographic variations in the use of tests for classifying peritoneal membrane function. The PET is the simplest procedure to perform and, as expected, has the most clinical experience related to its use. There are no data to suggest that one test is better than another in common clinical settings; hence, each center should use the test they are most comfortable with. The International Society for PD has recommended that a modified PET (3.86%/4.25% dextrose) dwell be used to optimally evaluate patients with ultrafiltration failure.218
Baseline peritoneal membrane transport characteristics should be established after initiating a daily PD therapy.
It is recognized that to optimize solute removal and ultrafiltration volumes, one must understand peritoneal physiological processes and know each patient's individual peritoneal membrane transport characteristics. This could be done by careful observations of such clinical parameters as blood pressure, volume status, physical examination findings, well-being, and serum chemistry test results, adjusting the peritoneal prescription as indicated. One is likely to be better able to do this if one documents peritoneal membrane transport characteristics in each patient. Once established, these data can be used to guide prescription writing and predict clearances and ultrafiltration volumes. Kinetic modeling programs have been developed that use peritoneal membrane transport test data from the standard PET to help in prescription management. These have been validated for clinical use.
Data suggest that it would be best to wait 4 to 8 weeks after starting dialysis therapy to obtain this baseline measurement.
The initial instillation of dialysate into the peritoneal cavity and the initiation of PD therapy is associated with mild changes in local cytokine production, peritoneal vascularity, and blood flow. These changes in peritoneal anatomy and perfusion potentially can influence peritoneal membrane transport. Historical data have suggested there is a small increase in D/P ratio for small solutes during the first month on PD therapy.219 This phenomenon recently was confirmed in a longitudinal analysis of 50 new PD patients.220 One-week, 1-month, and 1-year PET results from individual patients were compared. Significant changes in D/P urea (0.91 versus 0.94), D/P creatinine (0.55 versus 0.66), and end dialysate dextrose concentration over initial dialysate dextrose concentration (D/D0) glucose (0.38 versus 0.36) were noted. One-month PET results correlated better with 1-year results than did 1-week PET results. D/D0 values for glucose changed the least during the first month, and 1-week D/D0 values better predicted transport characteristics than 1-week drain value.
Based on these and historic data, it is recommended that the “baseline” peritoneal membrane transport study is obtained after the first 4 to 8 weeks of starting dialysis. During training, one could “estimate” peritoneal membrane transport rate by measuring the drain volume from a 4-hour dwell of 2.5% dextrose and comparing expected D/P ratios of creatinine to the patient's observed drain volume. However, as noted, the observed drain volume is not as predictive as other laboratory measurements. For most patients at the initiation of dialysis therapy, there is some RKF present. Therefore, estimating delivered clearance and ultrafiltration volumes from D/P ratio predicted by drain volumes observed during training suffice until a formal PET and 24-hour dialysate collection can be obtained. Standard clinical practice usually involves a timed 4-hour dwell with 2.5% dextrose during training and a follow-up PET at about 1 month after initiating PD therapy, at which time other issues regarding prescription management can be reviewed.
Peritoneal membrane transport testing should be repeated when clinically indicated (see Table 15).
In general, peritoneal transport is stable over time. However, small cohort studies that evaluated peritoneal transport characteristics over time, often with a short follow-up period, suggest that in some patients, peritoneal transport changes.221 Impaired ultrafiltration is the most frequent clinically noted abnormality. The prevalence of this change is dependent on dialysis vintage. One review using a clinical definition for ultrafiltration failure (defined as a need for hypertonic exchanges) suggested it was present in 3% of patients at 1 year and 31% after 6 years.222 In another cross-sectional study of patients on PD therapy for a median of 19 months (range, 0.3 to 178 months) and using a laboratory definition of ultrafiltration failure (ultrafiltration <400 mL after a 4-hour dwell with 4.25% dextrose), impaired ultrafiltration was noted in 23% of patients.216 It appears (from these studies in unselected patients) that over time, there tends to be an increase in transport manifested by higher MTACs, higher D/P ratios for small solutes, decrease in ultrafiltration when using glucose-containing fluids, and increased restriction to the transport of macromolecules.223 These clinical observations suggest there tends to be an increase in number of microvessels per unit of peritoneal surface area, along with decreased permeability to large-molecular-weight solutes. The net result is that the diffusive rate of solute transport tends to increase and drain volume/dwell tends to decrease. However, in many patients, total solute removal/dwell often remains stable because of these two offsetting phenomena.
As a result of the observed stability of peritoneal transport over time in most patients, one does not need to routinely document individual patients' peritoneal membrane transport characteristics over time with routine laboratory measurement (peritoneal membrane transport testing). However, one needs to clinically assess drain volume and clinical volume status in each patient on a regular basis. Drain volume can be assessed during a clinical visit by reviewing a patient's overnight (for CAPD) or daytime (for APD) drain volume and assessing the patient's need to use hypertonic dialysate solutions to maintain euvolemia. If one suspects a change in clinical status, peritoneal membrane testing should be repeated (see Table 15).
As noted, kinetic modeling programs have been developed that use peritoneal membrane transport test data from the standard PET to help in prescription management, and they have been validated for clinical use. As a result, most centers use the standardized PET as the baseline test to characterize peritoneal membrane transport. However, it now is recommended that one use a 3.86%/4.25% dextrose PET to work up a patient suspected to have ultrafiltration failure.218 Part of that evaluation includes comparison of current D/P data to historical baseline data. The 2.27%/2.5% dextrose PET and 3.86%/4.25% dextrose PET were compared, and no clinical differences between D/P ratios for such small solutes as creatinine were found.224 Two studies compared 2.27%/2.5% dextrose with 3.86%/4.25% dextrose PET. Forty stable PD patients were found to have little difference in D/P creatinine values, but expected differences in ultrafiltration profile.225 A subsequent study of 154 patients compared the 2 tests, found little clinical differences in D/P creatinine values, and established reference values for the 4.25% dextrose PET.226
These data suggest that in common clinical practice, one could compare D/P ratios for small-solute transport between tests. If ultrafiltration failure is suspected, the 3.86%/4.25% dextrose PET would be most useful, even if a 2.27%/2.5% PET was done at baseline.
All measurements of peritoneal transport characteristics should be obtained when the patient is clinically stable and at least 1 month after resolution of an episode of peritonitis.
Peritonitis is associated with peritoneal inflammation, which, in turn, is associated with hyperemia and changes in peritoneal transport. These changes usually are transient. The most striking clinical finding noted during an episode of peritonitis is impaired ultrafiltration.227 This is associated with an increase in peritoneal transport of low-molecular-weight solutes and increased rates of glucose absorption. These changes usually are transient and typically resolve within a month after resolution of the peritonitis.228,229
There have been no prospective randomized trials comparing patient outcomes with the use of various test methods. Therefore, one cannot be recommended over the other. However, it is unlikely there will be differences among test methods, and that study has not been recommended. These tests are designed to classify and evaluate membrane function. Although suggested by the literature that peritoneal transport type may influence patient outcome, it is controversial about whether patients with different baseline transport characteristics have different clinical outcomes or need to be on different types of PD therapies. Patients with all types of peritoneal function have been managed successfully on each of the different types of PD modalities (CAPD versus APD). The number of patients with ultrafiltration failure at any one center is limited, and data are just emerging on identifying them with use of 3.86%/4.25% dextrose PET. Therefore clinical data for outcomes after adjusting therapy based on PET findings and the use of newer PD fluids are lacking. With current therapies/solutions, it was shown that longitudinal laboratory monitoring of peritoneal transport of individual patients is not indicated. It is possible that if routine testing is no longer done as newer solutions are used, one may not have the data to evaluate longitudinal changes in transport and response to therapy with the use of these solutions.
Most centers are already using standard PET in clinical practice. Many are routinely monitoring transport changes over time (most on a yearly basis, although the prior KDOQI PD Adequacy Guidelines recommended more frequent monitoring). These CPRs are less demanding than the original KDOQI PD Adequacy Guidelines and—as CPRs instead of CPGs—should make implementation easier because there will be no related performance measures.
The frequency of testing has been decreased compared with prior KDOQI PD Adequacy Guidelines. They are similar to anticipated revisions of the Canadian and European guidelines for PD.