Appropriate interventions for access dysfunction may result in an increased duration of survival of the AVF.
5.1 Problems developing in the early period after AVF construction (first 6 months) should be promptly addressed.
- 5.1.1 Persistent swelling of the hand or arm should be expeditiously evaluated and the underlying pathology should be corrected. (B)
- 5.1.2 A program should be in place to detect early access dysfunction, particularly delays in maturation. The patient should be evaluated no later than 6 weeks after access placement. (B)
5.2 Intervention:
Intervention on a fistula should be performed for the presence of:
- 5.2.1 Inadequate flow to support the prescribed dialysis blood flow. (B)
- 5.2.2 Hemodynamically significant venous stenosis. (B)
- 5.2.3 Aneurysm formation in a primary fistula. Postaneurysmal stenosis that drives aneurysm also should be corrected. The aneurysmal segment should not be cannulated. (B)
- 5.2.4 Ischemia in the access arm (B).
5.3 Indications for preemptive PTA:
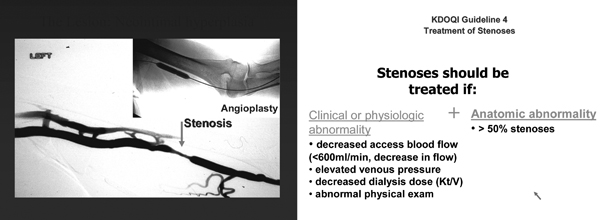
A fistula with a greater than 50% stenosis in either the venous outflow or arterial inflow, in conjunction with clinical or physiological abnormalities, should be treated with PTA or surgical revision. (B)
- 5.3.1 Abnormalities include reduction in flow, increase in static pressures, access recirculation preempting adequate delivery of dialysis, or abnormal physical findings. (B)
5.4 Stenosis, as well as the clinical parameters used to detect it, should return to within acceptable limits following intervention. (B)
5.5 Thrombectomy of a fistula should be attempted as early as possible after thrombosis is detected, but can be successful even after several days. (B)
5.6 Access evaluation for ischemia:
- 5.6.1 Patients with an AVF should be assessed on a regular basis for possible ischemia. (B)
- 5.6.2 Patients with new findings of ischemia should be referred to a vascular access surgeon emergently. (B)
5.7 Infection:
Infections of primary AVFs are rare and should be treated as subacute bacterial endocarditis with 6 weeks of antibiotic therapy. Fistula surgical excision should be performed in cases of septic emboli. (B)
Initial Problems (CPG 5.1)
Minor swelling normally is found postoperatively after placement of an AVF regardless of location and type of anastomosis. This “physiological” swelling disappears within the first week. Swelling of the hand or area of the fistula should be treated with hand elevation and patient reassurance. Because prevention is always preferable to therapy, a major aspect of preventing postoperative swelling is to rest the arm. Persistent swelling requires further attention to exclude major outflow obstruction. Hematoma, infection, and venous hypertension also should be excluded by clinical examination277,391,392; noninvasive ultrasound examination helps confirm extravasations and hematomas or purulent infiltrations, as well as strictures/stenoses of the venous outflow tract.45,124,393 Although angiography (fistulography) can show a venous stenosis causing venous hypertension, DDU is the preferred diagnostic method because it avoids any diagnostic cannulation of the newly created AVF and thereby avoids iatrogenic damage of the thin wall of the freshly arterialized vein. If a stenosis is found, it should be treated with a balloon angioplasty.
Persistent hand edema usually follows a side-to-side anastomosis for creating the fistula and invariably results from downstream stenosis forcing the flow through venous collaterals. This process can produce classic chronic venostasis with skin ulceration. The lesion should be treated early by ligation of the tributaries. If delayed healing of the wound is noted in patients, the surgical technique should be examined closely. The surgical technique to close the skin preferably should use degradable suture material in an exclusively subcutaneous position supported by externally applied sterile adhesive strips to minimize the thickness of the scar.
Risk for bleeding and hematoma formation is greatest in the early stages of use of a fistula and greater in brachiobasilic fistulae than other types of fistulae at the wrist or elbow.77 Manifestations of an infiltration or hematoma aside from the obvious discoloration and swelling include the presence of high-frequency bruit on auscultation and a difference in intravascular pressure on palpation.277,391,392 Because hematoma may lead to access loss,77 hematomas should be treated surgically if they are compromising the lumen of the arterialized vein (producing stenosis).388 In the absence of luminal compromise by physical examination or DDU, the access should be rested until the margins of the fistula are again well demarcated.
Proficiency in cannulating fistulae is suboptimal in the United States despite considerable efforts to remedy the situation.120,394-396 One can improve needle design to minimize trauma397 and develop methods to increase the efficiency of buttonhole development,398 but it is for naught if the fistula cannot be cannulated consistently without infiltrations. Because an inability to “be sure of the location” of the 2 lateral borders of the fistula contributes to miscannulation (particularly in those who are obese or have deep fistulae) and is manifested by so-called clot aspiration and because DDU is very precise in depicting the borders of vessels (see CPG 1),344,399,400 patients should be referred for access mapping and photography. A useful procedure is for the ultrasonographer to draw a map on the surface of the skin with a washable marker directly over the center of the lumen (or the 2 lateral borders), make a digital photo map of the fistula based on ultrasound, and send the photograph of the usable portion of the fistula access to the dialysis center. Alternatively, the access can be marked with indelible ink that permits the establishment of a series of subsequent successful puncture sites to demarcate the center of the vessels if the rotating-site system of cannulation is used (see CPG 3). These techniques both educate the staff and develop expertise and confidence. In addition, they should foster greater expertise in assessing fistulae during the first postoperative weeks for delayed maturation. Prospective studies are needed to demonstrate this opinion-based strategy.
The majority of fistula creations can be performed on an outpatient basis. A crucial element is the postoperative examination and surveillance follow-up that is scheduled by either the surgeon or a vascular access coordinator representing the interdisciplinary VAT. The primary purpose is to detect problems of maturation (see CPG 2). Although a variety of factors can produce maturation failure,86,123,125 a greater than 70% successful fistula access rate can be achieved, even among patients who have diabetes86,87,401,402 and women.84 In a multiple logistic regression analysis of 148 grafts (60% forearm, 40% elbow), predictive factors of early failure were distal location (adjusted odds ratio [aOR], 8.21; 95% confidence interval [CI], 2.63 to 25.63; P < 0.001), female sex (aOR, 4.04; 95% CI, 1.44 to 11.30; P = 0.008), level of surgical expertise (aOR, 3.97; 95% CI, 1.39 to 11.32; P = 0.010), and diabetes mellitus (aOR, 3.19; 95% CI, 1.17 to 8.71; P = 0.024).403 Much of the prevention of delayed fistula maturation must occur preoperatively (see CPG 1) through appropriate selection of arterial and venous vessels, as well as procedures most suitable for the individual patient. Although it is the vein that must dilate and accept higher flows, the artery must be healthy too. The resistive index of the artery used to construct the fistula is a strong predictor of early primary HD fistula failure.404 However, despite selection of the best available artery and vein, maturation failure can still occur. By combining venous diameter (>0.4 cm) and flow volume (>500 mL/min) during DDU evaluation within the first 4 months after access construction, one can predict the likelihood of maturing a fistula,72 ie, one that can be cannulated and provides sufficient blood flow for dialysis, with 95% certainty (19 of 20 fistulae). Women were less likely to have an adequate fistula diameter of 0.4 cm or greater: 40% (12 of 30) compared with 69% for men (27 of 39). However, of note, the accuracy of experienced dialysis nurses in predicting eventual fistula maturity was excellent at 80% (24 of 30).72 This is more reason to have a protocol for regular clinical examination in place in dialysis centers to teach the skills of physical examination (see CPG 4) to all staff members and assess the developing fistula and not focus on only the access in current use. A new fistula should be monitored regularly during the postoperative 4 to 6 weeks for swelling, hematoma, infiltration, wound healing, and failure to mature.
Intervention (CPG 5.2)
Inadequate Flow
A primary fistula should be revised when it is unable to sustain adequate HD blood flow, manifested by the inability to achieve the prescribed Kt/V within a reasonable HD duration. Low access blood flow has a major effect on the delivery of dialysis: inadequate blood flow may result in inadequate dialysis, thereby increasing patient mortality and morbidity.405,406 Impaired flow in fistulae is caused by impaired arterial inflow related to the site of cannulation. Location of the anatomic reason varies between arterial and venous lesions, as well as lesions within the anastomotic area.
Arterioatherosclerotic narrowing of the feeding artery with reduced flow and stenosis of the artery are found in an increasing portion of the elderly, patients with hypertension, and patients with diabetes. Therefore, careful preoperative evaluation should document data on anatomic and functional status of the arterial vasculature, including flow in the brachial artery (see CPG 1).
As stated, peripheral location of first fistula, female sex, diabetes mellitus, and, finally, surgical expertise are the main predictive factors of early fistula failure.72 Because it is known that arterial calcification in patients with diabetes is more pronounced in the wrist than elbow region,407 selection of a more proximally located site for creation of the AV anastomosis, eg, the proximal radial or beginning brachial artery in the proximal forearm, may be the better alternative. Inadequate flow in the area of the AV anastomosis is produced primarily by surgical factors. Two studies403,408 emphatically stressed that the early failure rate of fistula may be 3-fold greater when constructed by “occasionally” working access surgeons compared with experienced surgeons.
However, an initially adequate artery may become inadequate in time. Four of 40 patients had brachial artery lesions contributing to access dysfunction.409 In a larger series, 41 of 101 fistulae had arterial inflow lesions at the time of therapeutic intervention for dysfunction.268
In case of reduced flow caused by arterial inflow, 2 therapeutic options exist: stenosis of the feeding artery may require interventional angioplasty or surgical revision, or inadequate quality of the feeding artery (caused, eg, by calcification) may require a more proximally located new AV anastomosis. Although chronic arterial lesions in upper limbs bearing vascular access devices for HD most often manifest themselves as insufficient flow for HD treatment, the process may be severe enough to produce thrombosis and ischemia. For correcting stenoses, PTA is a safe and effective technique with a low rate of reintervention.268
Juxta-anastomotic venous stenosis is a commonly observed lesion. It occurs from the change in hemodynamic flow character from the artery into the vein and from devascularization of the venous wall during exposure, even after excellent surgery. Placement of the “arterial needle” downstream of this stenosis obviously supports the phenomenon of impaired flow. At times, it may be impossible to traverse the AV anastomosis by using the retrograde approach, and antegrade puncture of the brachial artery will be needed.410Although interventional procedures are successful with this type of lesion,411 construction of a new AV anastomosis (revision) at a more proximal location is the preferred procedure.112 However, the therapeutic strategy depends on the type of lesion and variability of local expertise.
Hemodynamically Significant Venous Stenosis
The commonly used parameter to characterize the hemodynamic relevance of a stenosis is a reduction in vessel diameter exceeding 50% based on angiographic and/or ultrasonographic findings. In contrast to an exact diagnosis in a synthetic AVG with a known standard diameter, it may be difficult to describe reliably the percentage of narrowing in a native vein, particularly because this vein may present a prestenotic and/or poststenotic aneurysmic enlargement. The hemodynamic relevance of a 50% stenosis in a native AVF therefore should be supported by clinical symptoms, abnormal physical findings, and flow measurements (see CPG 4). The diagnosis of “hemodynamically relevant venous stenosis” based on a combination of clinical and technical findings should initiate a corrective procedure, either percutaneous or surgical intervention.
In AVFs, significant stenoses may not elevate dynamic or static pressures, although such lesions can result in decreased access flow and elevated recirculation (see CPG 4) that are associated with increased risk for thrombosis.369 Treatment of hemodynamically significant venous stenosis prolongs the use-life of the AVF.322,356,358,369,412 A study of 32 patients and 30 controls showed a beneficial effect on AVF survival of prophylactic angioplasty of stenoses.390 Subsequent Kaplan-Meier analysis of a larger cohort of patients over 5 years showed that preemptive treatment decreased the failure rate (P = 0.003), and the Cox hazards model identified treatment (P = 0.009) and greater baseline access flow (P = 0.001) as the only variables associated with favorable outcome.389 A significant increase in access blood flow rate was observed, as well as a significant decrease in access-related morbidity by approximately halving the risk for hospitalization, central venous catheterization, and thrombectomy. This group showed, in a population of 120 patients with AVFs, that UDT measurements were reproducible and highly accurate in detecting stenosis and predicting thrombosis in forearm AVFs. Neither QA/MAP nor ∆QA improved the diagnostic performance of QA alone, although its combination with ∆QA increased the test's sensitivity for stenosis.339 These data support the value of monitoring and surveillance in AVFs (see CPG 4). In AVFs, 75% of stenoses producing low flow are at or near the AV anastomosis and 25% are in the outflow track.
Aneurysm Formation in a Primary Fistula
Progressive enlargement of an aneurysm eventually can compromise the skin above the fistula, leading to possible rupture. This can result in hemorrhage, exsanguination, and death. In the Work Group's opinion, large aneurysms can prevent access to the adjacent fistula for needle placement, thereby limiting potential cannulation areas.
Aneurysm formation in a primary fistula can be observed in the following situations:
Therapeutic options for managing the aneurysms include the following:
Fig 8. Treatment of stenosis. (Courtesy of Dr Thomas Vesely)
Indications for Preemptive PTA (CPG 5.3)
Preemptive PTA may be indicated in certain cases of abnormal physical findings (see Fig 8). These findings are more important than other criteria. See also the rationale for CPG 5.2. However, certain facets should be kept in mind. This may be particularly important in “underserved” areas where the dialysis staff has no choice other than to rely on abnormal physical findings.
Tools for physical examination have been described in CPG 4. However, Table 17 provides a quick summary.
To detect the early beginning of an abnormality requires continuous meticulous education and daily practice. When a high level of expertise is achieved, a definitive diagnosis can be achieved in approximately 60% to 80% of cases through the presence of abnormal physical findings that lead to an intervention. These findings should be documented and preserved in the chart and—if possible—electronically to continue the observation of the very earliest abnormality. In the remaining 20% to 40% of patients without a definitive diagnosis after physical examination, further diagnostic steps should be undertaken using (preferably first) ultrasound followed by, if necessary, angiographic techniques, including the option of angioplasty during the same session; however, this is dependent on local availability and expertise.
Previous Thrombosis in the Access
It was shown repeatedly that thrombosis of AVFs is caused by anastomotic disorders, predominantly stenosis. Episodes of hypotension during HD may be contributory in some cases. No data exist to determine whether hypotension alone, even if for a few hours, can produce thrombosis in the absence of an underlying stenosis limiting flow into the access. Irrespective of type of treatment given for the previous episode(s) of access thrombosis, these patients should be considered at risk because anastomotic residuals or recurrent development of stenosis at the same site are common. Therefore, special attention should be taken to prevent recurrence of clinical signs. This strategy requires repeated continuous physical examination—a quick chairside procedure in the hands of experienced personnel preceding any cannulation procedure.
Persistent Abnormal Surveillance Test (see CPG 4.2)
Because surveillance test results at times are observer dependent, an abnormal isolated finding in any case should be supported by abnormal physical symptoms. Persistence of abnormal physical findings and surveillance test results (elevated pressures, low flows, abnormal recirculation) require that further diagnostic steps be initiated to establish an exact diagnosis and lead to timely treatment (see CPG 4).
Stenosis (CPG 5.4)
In the absence of method errors, repeated failure to deliver the prescribed dialysis dose by using an AVF should result in immediate evaluation of the vascular access when other reasons can be excluded, eg, technical errors, timing errors, and so on. (See the Guidelines for HD Adequacy and also the rationales for CPG 5.1 and CPG 5.2.)
The degree of stenosis is graded by the percentage of narrowing of the access, the reference being the diameter of the immediately upstream or downstream “normal vessel.” The reference diameter can be difficult to determine when the AVF is irregular or aneurysmal or at the confluence of 2 vessels. Grading of severity also can be done on the basis of the drop-off in systolic or mean pressure across the stenosis.414,415 The degree of residual effacement tolerated varies among interventionalists. Some demand no residual at all unless it is the first PTA ever done. Swelling, local or generalized in the arm, caused by central venous stenosis may take additional time to resolve.
Dilation often is painful locally and local anesthesia may be needed at times. Venous stenosis in the outflow may be “rock hard” and require high-pressure balloons (bursting pressures of 25 to 30 atmospheres), as well as more prolonged inflation periods. Resistant stenoses are less common, usually less than 1% in forearm and 5% of upper-arm fistulae.112 There is no convincing proof that such lesions respond better to cutting ballons416 because studies have been small and not prospective. The Work Group recommends that high-pressure balloons be used first because cutting devices have not been studied adequately.
Thrombectomy (CPG 5.5)
In most patients, thrombosis is the final complication after a period of AVF dysfunction. Treatment of thrombosis should start as early as possible. The risk of delay is progressive growth of the thrombus that makes interventional/surgical procedures more difficult and risky with regard to long-term success. The vascular access should be reopened as soon as possible to resume regular dialysis treatment and avoid resorting to a short-term catheter. In addition, delay produces a longer period of contact between the surface of the thrombus and the vessel wall, thereby increasing the risk that extraction of thrombus may further damage the endoluminal layer. This could favor future thrombotic events. Early intervention increases the likelihood that the same AVF can be used to provide future dialyses.
Although thrombectomy procedures are more challenging in fistulae than grafts, results are more rewarding.417 Better long-term patency has been achieved in the largest series to date as long as the underlying stenoses are sufficiently dilated: 1-year primary patency rates of 50% and secondary patency rates of 80% have been reported.418 Results reported in the upper arm are not as good. The unmasking of stenoses in close to 100% of cases warrants stenosis-detection programs similar to those for grafts.419
After thrombosis is established, resolution depends on local expertise. Interventional thrombectomy and PTA of the underlying stenosis have gained wide acceptance. Nevertheless, there are no results from a larger series of surgical treatment of AVF thromboses available. This leads to the astonishing fact that there are no comparable data available in this important field of access care.
Thrombosed fistulae can be declotted by using purely mechanical methods (dilation and aspiration),419 a thrombolytic,420 or a combination of both.421 Success rates are greater than 90% for the techniques. If a central vein stenosis is found, interventionalists frequently resort to the use of stents. Long-term results after dilation in the largest series are better in forearm native fistulae compared with grafts. Initial success rates for declotting are better in grafts compared with forearm fistulae, but early rethrombosis is frequent in grafts; thus, primary patency rates can be better for native fistulae after the first month's follow-up.419 Although AVF function may be reestablished successfully as long as a week after thrombosis occurs, most should be treated as soon as possible.422
A variety of devices are available for mechanical thromboaspiration. With all, there are the issues of residual clots and cost-effectiveness of the devices over the simple procedure of catheter-directed aspiration. A meta-analysis should be performed.
Surgical thrombectomy is performed by using a Fogarty thrombectomy catheter, supported by retrograde digital expression of the thrombotic material and followed by correction of the stenosis by using a couple of techniques according to the individually varying condition. However, there are only scattered reports with initial success rates of only 65%423 compared with 90% or better for endovascular techniques. In a small study of 29 patients, a primary patency rate of 50% at 4 months was reported.424 Surgery seems to be the preferred technique to treat thrombosis in forearm AVFs with juxta-anastomotic stenoses, mainly by placement of a new anastomosis.424 With more proximally/centrally located thromboses, preference should be given to interventional endoluminal techniques. Early recurrence of stenosis/thrombosis can be decreased by insertion of a stent. On occasion, when both the artery and vein are thrombosed, conversion from a side-to-side to end-to-side anastomosis can be attempted, with the goal of using the newly created fistula immediately. This procedure was successful in 57% of 72 patients, particularly those with thrombosis of the AVF to the first side branch only, with the remaining fistula maintaining patency through collateral flow.425
Access Evaluation for Ischemia (CPG 5.6.1)
This evaluation should be a part of regular monitoring conducted routinely in all dialysis facilities. Particularly elder and hypertensive patients with a history of peripheral arterial occlusive disease and/or vascular surgery, as well as patients with diabetes, are prone to develop access-induced steal phenomenon and steal syndrome. In any case, clinical examination is mandatory, followed by ultrasound or radiological evaluation, as necessary. The patient must be referred to a vascular surgeon to decide on additional procedures. Delay can lead to catastrophic gangrene and hand amputation. The importance of this type of monitoring will increase in the future because of demographic changes in the dialysis population.
An AVF normally produces an alteration in blood flow patterns, a “physiological” steal phenomenon,426 that is seen in forearm AVFs and in a greater incidence in elbow/upper-arm AVFs.427 Physiological steal occurs in 73% of AVFs and 91% of AVGs.428 With the aging of the HD population and the increase in arterial changes caused by diabetes and hypertensive remodeling, the incidence of symptomatic peripheral ischemia to the hand/arm (pain, necrosis of ≥1 fingertips) is increasing, but fortunately is still uncommon (~1% to 4%).48 Milder symptoms of coldness and some pain during dialysis may occur in up to 10% of cases and fortunately improve over weeks to months.429 It also is more common with prosthetic bridge grafts; less than 2% versus 4%.48,430 A decrease in distal perfusion pressures is found regularly and is more pronounced in patients with advanced arteriomedial sclerosis. In this type of patient, occurrence of a steal syndrome seems less dependent on access flow volume than on degree of the peripheral arterial obstructive disease.
Recently, staging according to lower-limb ischemia was proposed48:
It is important to differentiate the findings of hand ischemia from those of carpal tunnel compression syndrome, tissue acidosis, and edema from venous hypertension. Noninvasive evaluation should be performed, including digital blood pressure measurement, DDU, and—if available—transcutaneous oxygen measurement.48
Corrective results may be good at an early point in the process, but in any of these patients, one should be aware that the process of arterial damage could be progressive. Particularly in older patients with diabetes with an elbow/upper-arm AVF, monomelic ischemic neuropathy can be observed; an acute neuropathy with global muscle pain, weakness, and a warm hand with palpable pulses starting within the first hours after creation of the AVF.431 Diagnosis of monomelic ischemic neuropathy is a clinical diagnosis, and immediate closure of the AVF is mandatory.
Emergent Referral to a Vascular Access Surgeon (CPG 5.6.2)
Although most ischemic manifestations occur early after surgery, in about a quarter of all patients, they can develop months to years after arterial constrictions. Fingertip necroses are an alarming symptom with an initially slow progression in most patients over weeks and a rapid final deterioration leading to necrosis and gangrene, indicating that one should aim for early intervention. If ischemic manifestations threaten the viability of the limb, the outflow of the fistula should be ligated.
Therapeutic options depend on the cause of steal syndrome. Arterial stenoses proximal to the anastomosis obstructing the arterial inflow may be dilated by angioplasty,411 but not in the case of advanced general arterial calcification. High-flow–induced steal syndrome requires a decrease in AVF flow volume. Banding procedures of the postanastomotic vein segment using different techniques as practiced in the past were not as successful as expected.432 It is more beneficial to decrease the diameter of the anastomosis or create a new AV anastomosis distally. The success of the procedure after surgery should be evaluated by using access flow measurements.
In cases in which a physiological steal phenomenon becomes clinically symptomatic, ligation of the peripheral limb of the radial artery may be successful. Clinically symptomatic steal syndromes with normal or low BFRs represent the majority of cases with access-related peripheral ischemia. Since the new technique of the distal revascularization—interval ligation (DRIL) operation was published in 1988,429 several groups have confirmed the good results.48,433 In patients with a venous anastomosis to the brachial artery, with the DRIL procedure, the anastomosis is bridged by a venous bypass, after which the artery is ligated closely peripherally to the anastomosis. BFR into the AVF does not change substantially. Most patients do significantly better, presumably because of an increase in peripheral arterial perfusion.
In patients with low BFRs and signs of peripheral ischemia, the proximal AV anastomosis technique provides satisfactory results.434 The idea is to ligate the preexisting anastomosis to the brachial artery in the region of the elbow or distal upper arm and place a new arterial anastomosis in the proximal upper arm, somewhere near the beginning of the subclavian artery. Blood volume is brought down to the vein through an interposed vein graft or small-diameter PTFE graft. Thus, a sufficient BFR into the vein is provided and peripheral perfusion pressure is reestablished; cannulation for HD can be continued immediately.
Infection (CPG 5.7)
Although infections of fistulae are rare, any episode of infection potentially is lethal in face of the impaired immunologic status of long-term dialysis patients.
Very rare access infections at the AV anastomosis require immediate surgery with resection of the infected tissue. Should an arterial segment be resected, an interposition graft using a vein can be attempted or a more proximal new AV anastomosis may be created with exclusive use of degradable suture material.
More often, infections in AVFs occur at cannulation sites. Cannulation at that site must cease, and the arm should be rested.
In all cases of AVF infection, antibiotic therapy is a must, initiated with broad-spectrum vancomycin plus an aminoglycoside. Based on results of culture and sensitivities, conversion to the appropriate antibiotic is indicated. Infections of primary AVFs should be treated for a total of 6 weeks, analogous to subacute bacterial endocarditis.435 A serious complication of any access-related bacteremia is represented by metastatic complications, as described.159