GUIDELINE 6. MARKERS OF CHRONIC KIDNEY DISEASE OTHER THAN PROTEINURIA
Markers of kidney damage in addition to proteinuria include abnormalities in the urine sediment and abnormalities on imaging studies. Constellations of markers define clinical presentations for some types of chronic kidney disease. New markers are needed to detect kidney damage that occurs prior to a reduction in GFR in other types of chronic kidney diseases.
- Urine sediment examination or dipstick for red blood cells and white blood cells should be performed in patients with chronic kidney disease and in individuals at increased risk of developing chronic kidney disease.
- Imaging studies of the kidneys should be performed in patients with chronic kidney disease and in selected individuals at increased risk of developing chronic kidney disease.
- Although several novel urinary markers (such as tubular or low-molecular weight proteins and specific mononuclear cells) show promise of future utility, they should not be used for clinical decision-making at present.
Abnormal urinary excretion of albumin and total protein (Guideline 5) is a highly sensitive indicator of glomerular disease. The results of urine sediment examination and of imaging studies of the kidney, however, can also suggest other types of chronic kidney diseases, including vascular, tubulointerstitial, and cystic diseases of the kidney. In addition, proteins other than albumin in the urine may indicate tubulointerstitial injury. At present, there are no clinically proven markers specific for tubulointerstitial or vascular diseases of the kidney. The purpose of this guideline is to review: abnormalities of urine sediment and abnormalities of imaging studies associated with kidney damage; the relationships of these abnormalities to clinical presentations of kidney disease; and possible new markers of kidney damage.
In some specific types of chronic kidney disease, abnormalities other than proteinuria are present prior to reduction in GFR. In general, urinalysis and ultrasound of the kidneys are helpful non-invasive tests to detect kidney damage. In addition, these assessments provide clues to the type (diagnosis) of chronic kidney disease.
Abnormalities of the Urinary Sediment
Examination of the urinary sediment, especially in conjunction with assessment of proteinuria, is useful in the detection of chronic kidney disease and in the identification of the type of kidney disease. Urinary sediment examination is recommended in patients with chronic kidney disease and should be considered individuals at increased risk of developing chronic kidney disease.
Cells may originate from the kidneys or from elsewhere in the urinary tract, including the external genitalia. Casts form only in the kidneys and result from gelation within the tubules of Tamm-Horsfall protein, a high molecular weight glycoprotein derived from the epithelial surface of the distal nephron. Casts entrap material contained within the tubular lumen at the time of cast formation, including cells, cellular debris, crystals, fat, and filtered proteins. Gelation of Tamm-Horsfall glycoprotein is enhanced in concentrated urine and at acidic pH levels. Examination of the urinary sediment for casts requires careful preparation. A “fresh” first morning specimen is optimal, and repeated examination may be necessary.
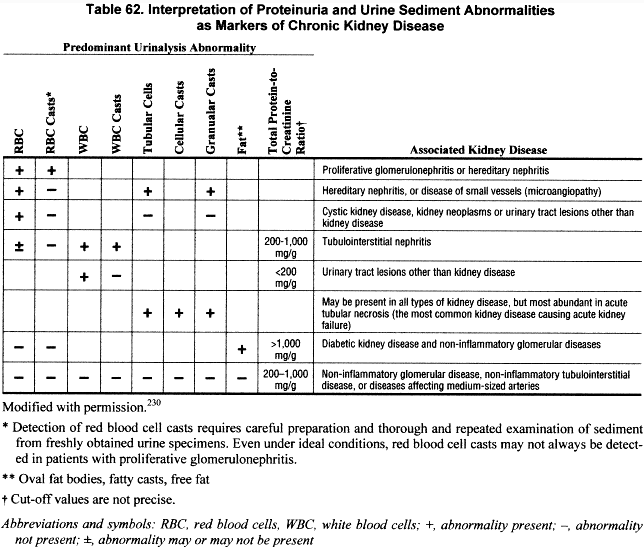
The presence of formed elements in the urinary sediment may indicate glomerular, tubulointerstitial, or vascular kidney disease. Significant numbers of erythrocytes, leukocytes, or cellular casts in urinary sediment suggest the presence of acute or chronic kidney disease requiring further work-up. The differential diagnosis for persistent hematuria, for example, is quite broad, including glomerulonephritis, tubulointerstitial nephritis, vascular diseases, and urologic disorders. Therefore, as with proteinuria, specific diagnosis requires correlation of urinalysis findings with other clinical markers. The presence of red blood cell casts strongly suggests glomerulonephritis as the source of hematuria. Dysmorphic red blood cells may also indicate a glomerular disease. Pyuria (leukocyturia)—especially in the context of leukocyte casts—may be seen in tubulointerstitial nephritis, or along with hematuria in various forms of glomerulonephritis. Urinary eosinophils have been specifically associated with allergic tubulointerstitial nephritis. Examination of a single urinary sediment may be adequate in most cases. However, the finding of a negative urinary sediment in patients considered to be at high risk for chronic kidney disease should lead to a repeat examination of the sediment. Table 62 provides a brief guide to the interpretation of proteinuria and abnormalities in urine sediment.
Urine dipsticks include reagent pads that are sensitive for the detection of red blood cells (hemoglobin), neutrophils and eosinophils (leukocyte esterase), and bacteria (nitrites). Thus, urine sediment examination is generally not necessary for detection of these formed elements. However, dipsticks cannot detect tubular epithelial cells, fat, or casts in the urine. In addition, urine dipsticks cannot detect crystals, fungi, or parasites. Urine sediment examination is necessary for detection of these abnormalities. The choice of urine sediment examination versus dipstick depends on the type of kidney disease that is being considered.
Imaging Studies
Abnormal results on imaging studies suggest either urologic or intrinsic kidney diseases. Imaging studies are recommended in patients with chronic kidney disease and in patients at increased risk of developing chronic kidney disease due to urinary tract stones, infections, obstruction, vesico-ureteral reflux, or polycystic kidney disease.
Hydronephrosis on ultrasound examination may be found in patients with urinary tract obstruction or with vesico-ureteral reflux. The presence of cysts—manifested either as multiple discrete macroscopic cysts or as bilaterally enlarged echogenic kidneys—suggests autosomal dominant or recessive polycystic kidney disease. Increased cortical echoes are a nonspecific but sensitive indicator of glomerular, interstitial, or vascular diseases. Imaging studies employing iodinated contrast agents can cause acute kidney damage and may present significant risks to some patients with decreased kidney function. The benefits of such studies must be weighed against potential risks. Baseline imaging studies will be appropriate in many patients. The appropriateness and frequency of follow-up studies will vary from case to case. Table 63 provides a brief overview of possible interpretations of abnormalities on imaging studies of the kidney.
Clinical Presentations of Kidney Disease
Some constellations of abnormalities in blood and urine tests or imaging studies comprise specific clinical presentations of kidney disease. These presentations are often not defined precisely in textbooks and review articles. The major features are described below. Table 64 defines these presentations according to level of GFR, markers of kidney disease (urine protein excretion, urine sediment examination, imaging studies), and other clinical features.
Decreased GFR and kidney failure are markers of more severe kidney disease (CKD Stages 2 through 5). The other presentations can occur without decreased GFR (CKD Stage 1) and can therefore serve as markers of kidney disease. Table 65 describes the most frequent presentations for each type of chronic kidney disease.
Decreased GFR and kidney failure. Either can be acute or chronic depending on duration, and due to any type (diagnosis) of kidney disease.
Nephritic and nephrotic syndromes. Nephritic syndrome (formerly “nephritis,” also termed “acute glomerulonephritis”) is an outdated term, characterized by hematuria with red blood cell casts, hypertension, and edema, with or without decreased GFR. Nephrotic syndrome (formerly “nephrosis”) is defined as total urine protein excretion in excess of 3,500 mg/d (equivalent to a total protein-to-creatinine ratio greater than approximately 3,000 mg/g), reduced serum albumin concentration, and edema, with or without decreased GFR. Both syndromes indicate the presence of a glomerular disease.
Tubular Syndromes. There are disorders resulting from abnormal tubule handling of water or solutes, without decreased GFR. They include diverse disorders such as renal tubular acidosis, nephrogenic diabetes insipidus, hyporeninemic hypoaldosteronism and other potassium secretory defects, renal glycosuria, renal phosphaturia, renal aminoaciduria, and many others. These syndromes often indicate a tubular interstitial disease.
Kidney disease with urinary tract symptoms. Most kidney diseases are asymptomatic, but in some tubulointerstitial diseases symptoms are associated with the kidneys or lower urinary tract. The most common causes include urinarytract infections, obstruction, and stones.
Asymptomatic urinalysis abnormalities. Abnormalities in urinary protein excretion or in urinary sediment without decreased GFR or urinary tract symptoms. Principal abnormalities include hematuria with red blood cell casts (due to glomerular diseases), pyuria with white blood cell casts, renal tubular cells, coarse granular casts, or non-nephrotic proteinuria.
Asymptomatic radiologic abnormalities. These include structural abnormalities of the kidney observed on imaging studies, without decreased GFR, urinary tract symptoms, or abnormal urinalysis.
High blood pressure due to kidney disease. Sustained elevation of arterial blood pressure as the result of disease of the parenchyma or major vessels of the kidney, with or without decreased GFR, but usually with either urinary abnormalities or radiologic abnormalities. Large vessel diseases (unilateral or bilateral) are included as chronic kidney diseases.
Strength of Evidence: New Urinary Markers
Increased urinary excretion of some low molecular weight (LMW) proteins and N-acetyl-![]() -D-glucosaminidase (NAG) are key diagnostic indicators in a number of specific tubular diseases and may identify patients at higher risk of GFR decline in other kidney diseases (Tables 66, 67, 68, and 69) (C).
-D-glucosaminidase (NAG) are key diagnostic indicators in a number of specific tubular diseases and may identify patients at higher risk of GFR decline in other kidney diseases (Tables 66, 67, 68, and 69) (C).
Low molecular weight proteinuria is a defining feature in several uncommon diseases of the kidney (Dent’s disease, autosomal dominant and cystinotic Fanconi syndrome, Lowe syndrome, Chinese herbs nephropathy).231 The urinary excretion of retinol-binding protein (RBP), but not albumin, increases with the presence of kidney scarring in reflux nephropathy in children.232 Increased urinary excretion rates of the LMW protein RBP and the tubular injury marker NAG are found in many patients with type I diabetes, even in the absence of albuminuria.38,42 Excretion of these markers appears to correlate with the degree of glycemic control in some studies,38,42 but not in others.233 In children with type I diabetes and normal albumin excretion, the presence of abnormal urinary NAG excretion at baseline indicates increased risk of developing microalbuminuria within 5 years (19.5% versus 0%, P < 0.05).234 In elderly patients with type 2 diabetes, individuals who developed macrovascular disease after 7 years of follow-up tended to have higher baseline NAG urinary excretion rates (P = 0.07).233Elevated urinary excretion of ![]() -2-microglobulin (>500 ng/min) at baseline predicted deterioration of kidney function over a mean follow-up period of more than 4 years in adult patients with membranous nephropathy.235 In adult and pediatric patients with a variety of kidney diseases (focal segmental glomerular sclerosis, membranous nephropathy, membranoproliferativeglomerulonephritis), a pattern of “very low” molecular weight proteinuria by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was associated with a higher rate of development of decreased kidney function at follow-up than was a pattern of “low” molecular weight proteinuria (50% versus 12.5%; P = 0.0001).236 In adult and pediatric patients with IgA nephropathy and normal kidney function at baseline, the presence of a low molecular weight pattern of proteinuria by SDS-PAGE at presentation was associated with an approximately 4-fold increase in their risk of developing a decreased GFR after 6 years of follow-up.237
-2-microglobulin (>500 ng/min) at baseline predicted deterioration of kidney function over a mean follow-up period of more than 4 years in adult patients with membranous nephropathy.235 In adult and pediatric patients with a variety of kidney diseases (focal segmental glomerular sclerosis, membranous nephropathy, membranoproliferativeglomerulonephritis), a pattern of “very low” molecular weight proteinuria by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was associated with a higher rate of development of decreased kidney function at follow-up than was a pattern of “low” molecular weight proteinuria (50% versus 12.5%; P = 0.0001).236 In adult and pediatric patients with IgA nephropathy and normal kidney function at baseline, the presence of a low molecular weight pattern of proteinuria by SDS-PAGE at presentation was associated with an approximately 4-fold increase in their risk of developing a decreased GFR after 6 years of follow-up.237
Urinary excretion of mononuclear cells may reflect the presence and/or degree of glomerular injury in some glomerular diseases, including diabetic nephropathy and IgA nephropathy (Table 70) (C).
In children with various kidney diseases, semiquantitative evaluation of urinary podocyte excretion correlated with the severity of mesangial proliferation, extracapillary proliferation, tubulointerstitial changes, and proteinuria.239 In pediatric patients with Henoch-Schönlein nephritis or IgA nephropathy followed for 12 months, the patients with resolution of podocyturia had the greatest resolution of acute inflammatory changes in their biopsies.240 In adult patients with biopsy-proven IgA nephropathy, the extent of active crescents correlated strongly with the number of CD14+ cells (macrophages) and CD56+ cells (NK cells) in urinary sediment.241
In adult patients with either clinically stable systemic lupus erythematosus (SLE) nephritis (WHO classes IIIa, b, IVb, c) or clinically active SLE nephritis (WHO classes IVb, c), only the patients with active disease showed evidence of podocyturia.242 In adult patients with type 2 diabetes, podocytes were present in the urine of 53% of microalbuminuric subjects and 80% of macroalbuminuric subjects, but in none of the normoalbuminuric subjects. Treatment with an angiotensin-converting enzyme inhibitor (ACE-inhibitor) reduced both urinary albumin excretion and podocyturia.243
The findings of hematuria, pyuria, and casts on urinalysis, or of cystic or echogenic kidneys on ultrasound, are well established as indicators of various chronic kidney diseases. In the proper setting, these findings are sensitive markers for the presence of chronic kidney disease, although they may not suggest a specific diagnosis. Since the novel markers described above (eg, low molecular weight proteinuria, mononuclear cyturia) have only been correlated with various chronic kidney diseases in a few studies to date, their application in clinical practice has not been established. In particular, inasmuch as these markers may correlate strongly with proteinuria, it is not certain that they can yet be considered independent indicators of disease or predictors of risk of disease progression.
In patients known to have chronic kidney disease on the basis of a decreased GFR, urinalysis and imaging studies may yield important diagnostic information. For example, the finding of red blood cell casts in the urine indicates a high likelihood of a proliferative glomerulonephritis. This finding would lead to a serological work-up and probably a kidney biopsy. The finding of diffuse nephrocalcinosis and nephrolithiasis on ultrasound in a patient with decreased GFR could suggest the possible diagnosis of hyperoxaluria, leading to specific blood tests.
In patients not previously known to have chronic kidney disease but presenting with symptoms suggestive of kidney disease (eg, edema, hematuria, or flank pain), examination of the urinary sediment may confirm the presence of kidney disease. Abnormalities in the sediment will be present in a large proportion of patients with chronic kidney disease. On ultrasound examination, the presence of a kidney stone and findings of obstruction may help to explain acute flank pain. Radiologic assessment may help to clarify other aspects of the nature of the kidney involvement. For example, bilateral small echogenic kidneys in a patient presenting with newly detected decreased kidney function can suggest a chronic rather than an acute process.
Examination of the urinary sediment may lead to the detection of kidney disease in patients presenting for evaluation of symptoms related to other organ systems. The evaluation of the urine in patients with signs of vasculitis or with carcinomas may result in detection of associated kidney disease. Findings suggestive of kidney disease may be expected to occur frequently in the evaluation of individuals presenting with hypertension, especially younger individuals.
In selected individuals with a normal GFR, but known to be at risk of chronic kidney disease, markers may serve as screening tests. For example, a patient at risk on the basis of a positive family history of polycystic kidney disease should undergo a screening kidney ultrasound one or more times before adulthood. See Guideline 3.
Application of the newer urinary markers (mononuclear cells and specific proteins such as NAG) described herein must await their validation in more extensive clinical studies.
Novel and expanded uses of established methodologies (such as Doppler or functional MRI) should be pursued in clinical research studies. Several novel urinary markers show promise of noninvasive demonstration of kidney damage or prediction of disease progression. None appears to be ready at this time for widespread application in clinical practice. Longitudinal and follow-up studies are necessary to verify whether abnormal NAG and possibly retinol-binding protein excretion in normoalbuminuric diabetic patients reliably predict later development of microalbuminuria and diabetic nephropathy. Similar studies are needed to confirm whether increased ![]() -2-microglobulin excretion predicts development of kidney failure in patients with idiopathic membranous nephropathy. Longitudinal studies of urinary excretion of specific cell types (macrophages, NK cells, podocytes) in diabetic nephropathy, Henoch-Schönlein nephropathy, and IgA nephropathy are also necessary in order to confirm preliminary findings that cyturia is strongly associated with activity in these diseases. Preliminary work on the urinary excretion of podocyte-specific marker proteins such as podocalyxin and nephrin should be validated by further studies.
-2-microglobulin excretion predicts development of kidney failure in patients with idiopathic membranous nephropathy. Longitudinal studies of urinary excretion of specific cell types (macrophages, NK cells, podocytes) in diabetic nephropathy, Henoch-Schönlein nephropathy, and IgA nephropathy are also necessary in order to confirm preliminary findings that cyturia is strongly associated with activity in these diseases. Preliminary work on the urinary excretion of podocyte-specific marker proteins such as podocalyxin and nephrin should be validated by further studies.