Traditional risk factors—such as diabetes, hypertension, dyslipidemia—and those specific to dialysis patients (anemia and mineral metabolism abnormalities) require regular assessment and treatment as per current recommendations. The relative importance and weight of each of these risk factors in the dialysis population is not known and, in the absence of controlled trials in this population, current recommendations from existing organizations should be followed, with special consideration given to potential risks.
Furthermore, lifestyle issues such as smoking, physical activity, depression, and anxiety are the cornerstones of therapy as in the general population. The treatment options are often similar, but the impact of these factors is potentially more profound in dialysis patients. These factors are all discussed in this section. Special attention will be paid to the difference between the usual recommendations and those for dialysis patients.
Guideline 16: Arterial Stiffness, Vascular and Valvular Calcification, Calcium, Phosphorus and PTH
The role of abnormalities of calcium, phosphorus, and PTH in contributing to arteriosclerosis, subsequent arterial stiffness, calcification and cardiac valve calcification is an area of intense research. The importance of these parameters to CVD outcomes and the biological plausibility of these variables in CVD processes require attention to them as “uremia-related” risk factors.
16.1 All dialysis patients should have pulse pressure (PP) determined monthly before dialysis.
16.1a For PP >60 mm Hg and systolic blood pressure >135 mm Hg, it is recommended that PP be reduced by achieving ideal body weight and the use of antihypertensive medication with the target PP being 40 mm Hg. (B)
16.2 Identification and treatment of calcification:
16.2a If arterial calcification is identified by plain radiography in any of the following sites (abdominal aorta, carotid arteries, ileo-femoral axis or femoro-popliteal axis), identification of calcification at another site should be sought. (C)
16.2b If vascular calcification is present in two or more sites, consideration should be given to prescription of a non-calcium-containing phosphate binder. (B)
16.3 All dialysis patients should follow current KDOQI Guidelines for treatment of calcium, phosphate, and PTH.78
16.3a Serum phosphorus should be maintained between 3.5–5.5 mg/dL (1.13–1.78 mmol/L). (B)
16.3b PTH should be measured every 3 months using an intact PTH assay (first-generation immunoradiometric assay). (C)
16.3bi For prevention of CVD, the target PTH value should be between 150–300 pg/mL (16.5–33.0 pmol/L). (B)
16.3bii Treatment strategies for PTH values <150 pg/mL (16.5 pmol/L) and >300 pg/mL (33.0 pmol/L) should be developed according to the KDOQI Bone Disease Guidelines.78(B)
Arterial stiffness (Weak)
Increased arterial stiffness in dialysis patients is the result of chronic flow/volume overload, uremia-induced endothelial dysfunction, fibroelastic intimal thickening, increased extracellular matrix, and medial calcification.334 Arterial stiffness may cause CVD because it increases LV after-load and decreases the diastolic pressure, resulting in a decrease in coronary perfusion. Arterial stiffness can be assessed by measurement of PWV using B-mode ultrasonography. However, pulse wave velocity is not easily measured in clinical practice. In contrast, PP can be easily measured and is an attractive surrogate for PWV. Pulse pressure, the difference between systolic and diastolic blood pressure, reflects LV ejection and aortic elasticity. Similar to PWV, increased PP has been associated with increased all-cause mortality in nondiabetic HD patients.188 Others have reported that pre- and post-dialysis blood pressures have independent associations with mortality in a manner that implicates wide pulse pressures.130(Moderately Strong)
Patients who had a decrease in PWV with a decrease in blood pressure also had regression in LVH.335 Further, it has been shown that patients whose PWV could be decreased by correcting hypertension had better survival rates than those whose PWV did not change with blood pressure decrease.195 Whether a decrease in PP would also identify those with better survival is not known. In a study of 22 HD patients with type 2 diabetes, there was an improvement in PWV among patients treated with fluvastatin.336 Whether the improved PWV associated with statins would improve cardiovascular outcomes requires further study. (Weak)
The evidence supporting an association between arterial stiffness and increased risk of death in dialysis patients is derived from prospective cohort studies using PWV as the estimate for arterial stiffness. A study of 243 chronic HD patients with a PWV >12.0 mL/sec compared to <9.4 mL/sec had an odds ratio of 5.4 for all-cause mortality and 5.9 for cardiovascular mortality.190 The evidence supporting an association between pulse pressure and mortality in dialysis patients is based on two prospective cohort studies. The first study cohort consisted of 1,243 chronic HD patients followed for 9 years.188 During the mean follow-up of 76 months, the mortality rate among those with PP <59 mm Hg was 28%, compared to 38%, 46%, and 60% for those with PP 60–79, 80–99 and >100 mm Hg respectively. Using multivariate analysis for nondiabetic patients, there was an 8% increase in the relative risk for all-cause mortality associated with each 10 mm Hg increase in pulse pressure. The second study cohort consisted of a random sample of 11,142 subjects followed from 1994–2000.130 Higher systolic and lower diastolic blood pressure were associated, in a multivariate analysis, with an increased risk of death. The associations are strong and consistent. (Moderately Strong)
Vascular calcification
The calcium content in coronary arteries of dialysis patients is much higher than that found in age-gender-matched controls and in nonuremic patients with CAD.337 Moreover, there is an association between the Agatston338 score and the prevalence of atherosclerotic disease in HD patients.339 However, the ACC/AHA did not recommend EBCT for the diagnosis of obstructive coronary disease due to the low specificity of this test.340 However, EBCT remains a valuable surrogate outcome when used in the tightly controlled environment of the randomized clinical trial. For clinical practice, spiral CT may be a feasible alternative341 but there are insufficient data to recommend routine use of this technique. (Weak)
A valid and reproducible estimate of vascular calcification that is easily applied in clinical practice is required. The method of Guerin342 uses ultrasound and soft-tissue radiographs to estimate arterial calcification in the common carotid arteries, abdominal aorta, iliofemoral axis and femoro-popliteal axis. The overall score ranges from 0–4 based on the number of calcified sites. Good inter-observer reproducibility has been reported. It is a valid predictor of all-cause mortality and cardiovascular mortality.343 However, the Work Group felt that the experience with this technique was limited, and that there would be major barriers to its acceptance and implementation. (Weak)
The recommendation made by the KDOQI Clinical Practice Guidelines for Bone Metabolism and Disease in CKD was that a non-calcium-based binder be used if there was evidence for severe vascular calcification.78 While the diagnosis is based on an incidental finding of vascular calcification on plain radiography, no suggestion is made for frequency of diagnostic evaluation nor is there a definition for severe vascular calcification.
Young dialysis patients with detectable coronary artery calcification had a mean daily calcium carbonate dose of 6,456 mg compared to 3,325 mg among those who had no detectable calcium.32 Older age, male gender, diabetes, dialysis vintage, higher serum calcium, and higher serum phosphorus have been associated with higher coronary artery calcification scores.339 Prevention of hyperphosphatemia is critical, but dietary phosphorus restriction and conventional HD are often not adequate. An orally administered phosphate binder is required. An alternative to calcium-based phosphate binders is desirable. One currently available alternative is sevelamer hydrochloride. In a randomized clinical trial, subjects treated with sevelamer for 12 months did not have a statistically significant increase in the coronary artery or in the aortic calcification scores while those treated with calcium carbonate or calcium acetate had a continued increase.81 The serum phosphorus values were well controlled in both groups. The incidence of hypercalcemia (16% vs. 5%) and the prevalence of an undesirable suppression of PTH at the end of the study (57% vs. 30%) were greater in the calcium-treated group. (Moderately Strong)
The calcification score at which to initiate treatment with a non-calcium-based phosphate binder is unclear. Ideally, calcium-based binders should be avoided entirely, but there are cost considerations as the currently available non-calcium-based binder (sevelamer hydrochloride) is considerably more expensive. The Work Group recommends that, if vascular calcification is noted in one part of the vascular tree (either carotids, aorta, ileo-femoral or femoropopiteal) and the calcium-phosphorus product exceeds 55, plain radiographs of the other areas should be made. If positive in one other area, a non-calcium-based phosphate binder should be considered. It is recognized that vascular calcification on plain radiographs does not disqualify arteriosclerosis (which may respond to reduction in calcium-phosphorus product) from atherosclerosis (which is unlikely to respond). (Weak) (Table 16)
Lanthanum carbonate is a non-calcium-based phosphate binder but there are insufficient published data on its efficacy and safety.344
Valvular calcification
Valvular calcification was found to be much more common in CKD patients treated with HD than in age-matched and gender-matched controls.345 There is an increased prevalence of valvular insufficiency with calcification of the mitral (29% vs. 6%) and aortic valves (22% vs. 6%). Using EBCT, calcification was reported in 45% of the mitral and 34% of the aortic valves in HD patients, compared to an expected 3%-5% in controls.339 Others found significant increases in valvular calcium estimated by EBCT over a 12-month period.337 There is no convincing evidence linking valvular calcification to abnormalities in serum calcium, phosphorus, or PTH. Valvular calcification is associated with a worse survival, perhaps mediated by increased LVH, for those with aortic valve calcification. There is also an increased risk for death among PD patients with valvular calcification (Table 17).346(Weak)
Serum phosphorus
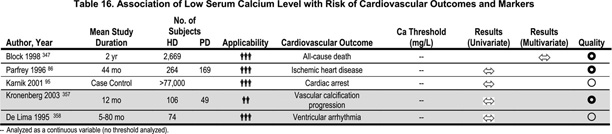
Observational studies in HD patients show a statistically significant increase in the risk for all-cause and cardiovascular mortality with serum phosphorus >6.5–6.6 mg/dL (2.10–2.13 mmol/L) (Table 18).347,348 The evidence linking increased serum phosphorus to vascular calcification is based on the observation that vascular calcification is an active process of ossification.349 Prior to the deposition of calcium in medial smooth muscle cells, bone matrix proteins are detectable. The linkage of hyperphosphatemia to development of vascular calcification is based on the observation that phosphorus can induce the production of bone-forming proteins in the vascular smooth muscle.350 The in vitro evidence for the role of inorganic phosphorus in the pathogenesis of vascular calcification has been reviewed.351 Exposure of cultured human aortic smooth muscle cells to concentrations of phosphorus similar to those found in CKD patients increased the expression of osteogenic factors. An additional mechanism by which hyperphosphatemia might cause cardiac disease is increased cardiac fibrosis.352 The progression of vascular calcification in coronary arteries has been associated with high doses of calcium-based phosphate binders32 and there is progression in patients prescribed a mean dose of 1,500 mg elemental calcium daily.81(Moderately Strong)
The strategies to treat hyperphosphatemia and the evidence for the use of non-calcium-based phosphate binders are described in the KDOQI Bone Disease Guidelines.78
PTH
It is common to consider hyperparathyroidism as a traditional risk factor for CVD (Table 19). The relative risk for all-cause mortality was 1.18 in the quintile with PTH values >511 pg/mL (56.2 pmol/L) compared to the referent quintile of PTH values 34–91 pg/mL (3.7–10.0 pmol/L) in an observational study.347 In another study, the relative risk for sudden death was 1.06 in the quintile with PTH values >496 pg/mL (54.6 pmol/L) compared to the reference quintile with PTH values 91–197 pg/mL (10.0–21.7 pmol/L).348(Weak)
Patients with histological evidence for adynamic bone disease have decreased ability to buffer exogenous calcium loads than do patients with high-turnover bone disease or those with mixed uremic osteodystrophy.353 Intact PTH has been used as a surrogate marker for bone metabolic activity. In PD patients, a prevalence of 63.2% for biopsy-proven low-turnover bone disease was reported.354 An intact PTH value <200 pg/mL (22.0 pmol/L) has been used to define relative hypoparathyroidism in HD patients.355(Weak)
Factors suppressing PTH include hypercalcemia, increased vitamin D levels, diabetes mellitus and increasing age. Despite being associated with these risk factors for CVD, hypoparathyroidism was found to be an independent predictor of mortality.355 In several studies, low PTH levels do not show a convincing association with a variety of markers of cardiovascular outcomes.
There are several intact PTH assays available, the most frequently used currently being the Nichols assay. This assay measures both active PTH and PTH fragments which might be either inactive or inhibitory. This assay has been used in the majority of studies reported in the literature. The measurement of serum PTH and the target values for CKD patients are discussed in the KDOQI Bone Disease Guidelines.78
Arterial stiffness
Serum phosphorus
Arterial stiffness
Serum phosphorus
PTH