The number of patients with chronic kidney disease (CKD) is increasing. Unfortunately, the survival of CKD patients remains poor.5 This is, in large part, due to premature cardiovascular disease (CVD) that manifests itself as coronary heart disease (CHD), cerebrovascular disease, and/or peripheral vascular disease (Table 1). There are 2 major overlapping categories of CVD: (1) disorders of cardiovascular perfusion, which include atherosclerotic CVD (ACVD); and (2) disorders of cardiac function, such as heart failure and left ventricular hypertrophy. Some risk factors are unique to each category of CVD, and some risk factors are shared by both categories of CVD.
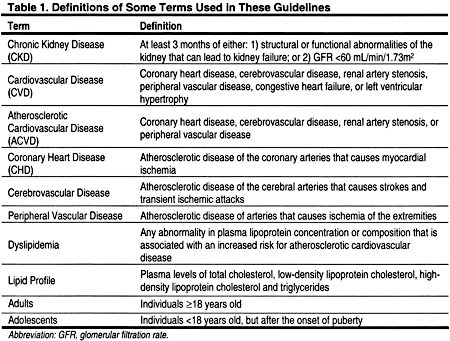
The National Kidney Foundation (NKF) Task Force on CVD concluded that the incidence of ACVD is higher in patients with CKD compared to the general population (Fig 1). 4 The Task Force concluded that patients with CKD should be considered to be in the highest risk category, ie, a CHD risk equivalent, for risk factor management.
Fig 1. The evolution of National Kidney Foundation guidelines for the management of dyslipidemias in patients with chronic kidney disease. To convert serum creatinine from mg/dL to mmol/L, multiply by 88.4. Abbreviations: Cr, serum creatinine; ESRD, end-stage renal disease; NCEP, National Cholesterol Education Program; CVD, cardiovascular disease; GFR, glomerular filtration rate; CKD, chronic kidney disease; KDOQI, Kidney Disease Outcomes Quality Initiative.
In response to recommendations of the NKF Task Force on CVD, the NKF Kidney Disease Outcomes Quality Initiative (KDOQI) convened a Work Group to develop guidelines for the management of dyslipidemias, one of the risk factors for CHD in CKD. The Work Group first met on November 27, 2000.
During the development of these guidelines, the NKF KDOQI also completed guidelines on CKD.2 These CKD guidelines defined CKD, and reiterated that CKD should be considered a CHD risk equivalent and that risk factors should be managed accordingly (Fig 1). In the CKD guidelines, CKD is defined according to the presence, for at least 3 months, of either of the following:
The definitions of Stages 1–5 CKD are based on measured or estimated GFR (Table 2), where the GFR is estimated from the serum creatinine using an established formula, as described in the KDOQI CKD Guidelines.2 Stage 1 CKD is defined by estimated GFR ≥90 mL/ min/1.73 m2, with evidence of kidney damage (as defined above). Stage 2 CKD is defined as evidence of kidney damage with mildly decreased GFR of 60–89 mL/min/1.73 m2. The level of estimated GFR, with or without kidney damage, defines Stages 3–4. Stage 5 (kidney failure) is defined as GFR <15 mL/min/1.73 m2, or the clinical indication for kidney replacement therapy with maintenance hemodialysis, peritoneal dialysis, or transplantation (Table 2). Thus, some Stage 5 patients may need kidney replacement therapy because of uremic symptoms, even when, based solely on GFR, they would be classified in Stage 4 (GFR 15–29 mL/min/1.73 m2) (Table 2).
Of the traditional risk factors for ACVD in patients with CKD, dyslipidemias may play a major role. In developing these guidelines, the Work Group was greatly aided by the National Cholesterol Education Program Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults, Adult Treatment Panel III (ATP III),3 and the National Cholesterol Expert Panel on Children (NCEP-C).6 The definitions of dyslipidemias adopted by the Work Group were those of ATP III (Table 3). In the end, the major task of the Work Group was to decide how the ATP III and NCEP-C guidelines should be applied to patients with CKD.
There is evidence from observational studies that, in addition to dyslipidemias, some "non-traditional" risk factors such as calcium, phosphorus, and parathyroid hormone,7,8 homocysteine,9–16 and systemic inflammation17–21 may also play a role in the pathogenesis of CVD in patients with CKD. However, unlike dyslipidemias, there are no intervention trials from patients in the general population (or in those with CKD) demonstrating that the modification of these non-traditional risk factors reduces CVD. Therefore, these guidelines focus on the assessment and treatment of dyslipidemias in patients with CKD. Unfortunately, there are no randomized controlled intervention trials in CKD patients showing that the treatment of dyslipidemias reduces the incidence of ACVD. Moreover, it is possible that trial results from the general population may not be applicable to all patients with CKD. It is also possible that in some subpopulations of CKD patients, treatment of dyslipidemias may not be as safe—or as effective—in reducing the incidence of ACVD, as it is in the general population. This may be due to the unique complications of CKD (eg, anemia, calcium and phosphorus metabolic abnormalities) that may contribute to the risk of ACVD in CKD. Therefore, the Work Group concluded that additional, randomized, placebo-controlled trials are urgently needed in patients with CKD, and that the use of a placebo is justified in the context of an appropriately designed trial, even when lipid levels fall within the treatment thresholds recommended by these guidelines (see IV. Research Recommendations).
One of the first tasks of the Work Group was to define the target population for guidelines on the management of dyslipidemia. It was decided to include all patients with Stage 5 CKD, as well as kidney transplant recipients, irrespective of whether kidney transplant recipients were classified as having CKD according to the KDOQI CKD Guidelines. Some kidney transplant patients who have normal kidney function (GFR ≥90 mL/min/1.73 m2) may not have CKD according to the KDOQI Guidelines defining CKD. Similarly, some patients with GFR ≥60 mL/min/1.73 m2 may not fit the KDOQI definition of CKD, because they do not have evidence of kidney damage, ie, they may have normal urine protein excretion, urine sediment, histology, and radiographic imaging. However, the CKD guidelines consider such patients at increased risk for CKD. As such, the Work Group decided to consider that all kidney transplant recipients have CKD, or are at increased risk for CKD, and to include kidney transplant recipients in the target population.
The Work Group considered whether the guidelines should include patients with Stages 1–4 CKD (GFR ≥15 mL/min/1.73 m2). These patients have a very high prevalence of dyslipidemias and ACVD,22–25 and it was concluded that the recently updated guidelines of the ATP III3 were generally applicable to patients with Stages 1–4 CKD. Specifically, it was concluded that it is unlikely that the recommendations of the ATP III would need to be modified for patients with Stages 1–4 CKD, except to:
Classification of CKD as a CHD risk equivalent has already been recommended by two NKF Work Groups.2,4 Therefore, for Stage 1–4 CKD patients, the Work Group focused its attention on the latter three issues, and otherwise recommended that the ATP III Guidelines be followed in all patients with Stages 1–4 CKD.
Finally, the Work Group considered whether to include children and adolescents in these guidelines (Fig 2). Although the ATP III covers only individuals ≥20 years of age,3 it was concluded that CKD individuals 18–20 should also be included and considered as adults.
Fig 2. Ages covered by the current guidelines, and those covered by previous guidelines developed for use in the general population.
Much has changed in the decade since the report of the NCEP-C.6 However, there are still very few studies of dyslipidemias in children and adolescents, either in the general population or in persons with CKD. In the end, it was concluded that adolescents (defined by the onset of puberty), in any stage of CKD or with a kidney transplant, should be included in these guidelines. Children (before the onset of puberty) should be managed according to existing guidelines, such as the NCEP-C.6
The Work Group also considered the recommendations of the NKF Task Force on CVD concerning the management of risk factors other than dyslipidemias.4 There are 2 potential reasons to assess other risk factors for ACVD: (1) to categorize overall risk for the purpose of making decisions regarding the management of dyslipidemia; and (2) to identify modifiable risk factors other than dyslipidemia that should also be treated. The first reason was considered unnecessary (for the purpose of these guidelines) by accepting the recommendation that a patient with CKD should be considered to have a CHD risk equivalent when deciding the appropriate management of dyslipidemia. However, the Work Group acknowledged that other risk factors are also important in the pathogenesis of ACVD and should be treated. Therefore, the Work Group concluded that for patients with CKD:
The task of the Work Group was greatly facilitated by the ATP III,3 and the NCEP-C for children and adolescents.6 However, the ATP III and NCEP-C make few specific recommendations for the evaluation and treatment of dyslipidemias in CKD, and none of the guideline statements includes or excludes patients with CKD. The ATP III notes that nephrotic syndrome is a cause of secondary dyslipidemia, and suggests consideration be given to the use of cholesterol-lowering drugs if hyperlipidemia persists despite specific treatment for kidney disease. The ATP III also notes that various dyslipidemias have been reported in persons with kidney failure. However, the ATP III suggests that a cautious approach be taken, since these persons are prone to drug side-effects, eg, they are at increased risk for myopathy from both fibrates and statins. In fact, the ATP III suggests that chronic kidney failure is a contraindication to fibrates.
The Work Group concluded that, in most areas, the ATP III and NCEP-C were applicable to adults and adolescents, respectively. It considered its principal task to define areas where the ATP III and NCEP-C needed modification and refinement for patients with CKD. In the end, relatively few modifications were needed (Tables 5 and 6).
These guidelines are intended for use by physicians, nurses, nurse practitioners, pharmacists, dietitians, and other health-care professionals who care for patients with CKD. The information contained in these guidelines can and should be conveyed to patients and their families in an understandable manner by their physician and/or other health-care professionals. The development of educational support materials designed specifically for patients and their families should be part of the implementation of these guidelines.
All guidelines should be updated whenever new, pertinent information becomes available. To anticipate when these guidelines may need to be updated, the Work Group discussed ongoing clinical trials in the general population and in patients with CKD, as those results may be pertinent to some recommendations. Late in the course of development of these guidelines, the results of the Heart Protection Study were published.34 This study randomly allocated 20,536 adults with coronary artery disease to simvastatin 40 mg versus matching placebo. Patients treated with simvastatin had an 18% reduction in coronary deaths. Importantly, the reduction in mortality was seen irrespective of the baseline level of cholesterol. This raised the possibility that all patients with known coronary artery disease should be treated with a statin, regardless of the serum cholesterol level. Ultimately, these and other results from ongoing trials could conceivably change the recommended approach to treatment of dyslipidemias. Some other important ongoing trials in patients from the general population include:
Some important, ongoing trials being conducted in patients with CKD include:
Thus, a number of potentially important trials will be completed within the next 3–5 years. Given the potential for these and other studies to provide information pertinent to the assessment and treatment of dyslipidemias in patients with CKD, it was concluded that these guidelines should be updated in about 3 years from the time of publication, and sooner if new, pertinent information becomes available before then. The Work Group will monitor the progress of these trials and recommend updating these guidelines as indicated.
Guideline Development
These guidelines were developed using 4 basic principles set forth by the KDOQI:
The guidelines were developed using an evidence-based approach similar to that endorsed by the Agency for Health-Care Research and Quality. The Work Group reviewed all pertinent, published evidence, and critically appraised the quality of studies and the overall strength of evidence supporting each recommendation.
Table 7. Rating the Strength of Recommendations. |
||||||||
|
Health outcomes are health-related events, conditions, or symptoms that can be perceived by individuals to have an important effect on their lives. Improving net health outcomes implies that benefits outweigh any adverse effects.
Rating the Strength of Guidelines and Evidence
The overall strength of each guideline statement was rated by assigning either "A," "B," or "C" (Table 7). An "A" rating indicates "it is strongly recommended that clinicians routinely follow the guideline for eligible patients. There is strong evidence that the practice improves net health outcomes, and benefits substantially outweigh harms." There were no guidelines that were assigned an "A" level recommendation. The "B" rating indicates "it is recommended that clinicians routinely follow the guideline for eligible patients. There is moderate evidence that the practice improves net health outcomes." A "C" rating indicates "it is recommended that clinicians consider following the guideline for eligible patients. This recommendation is based on either weak evidence, poor evidence or on the opinions of the Work Group and reviewers, that the practice might improve net health outcomes."
The strength of evidence was assessed using a rating system that takes into account (1) methodological quality of the studies; (2) whether or not the study was carried out in the target population, ie, patients with CKD, or in other populations; and (3) whether the studies examined health outcomes directly, or examined surrogate measures for those outcomes, eg, improving dyslipidemia rather than reducing CVD (Table 8). These 3 separate study characteristics were combined in rating the strength of evidence provided by pertinent studies.
Literature Retrieval and Review
The Work Group collaborated with a professional Evidence Review Team to identify and summarize pertinent literature (see Appendix 1). The Work Group and the Evidence Review Team first identified the topics to be searched, and the Evidence Review Team conducted the literature search. The topics that were selected for search included the incidence or prevalence of dyslipidemia, the association of dyslipidemia with ACVD, and the treatment of dyslipidemia in patients with Stage 5 CKD (including kidney transplant recipients). For patients with Stages 1–4 CKD, topics for the literature retrieval were limited to adverse effects of dyslipidemia treatment, the effects of dyslipidemia treatment on kidney disease progression, and the effects of therapies that reduce proteinuria on dyslipidemias. Systematic searches for all studies on dyslipidemia prevalence, association with ACVD, and treatment for patients with Stages 1–4 CKD were not conducted. As described above, the Work Group concluded a priori that the ATP III Guidelines were generally applicable to patients with Stages 1–4 CKD.
Briefly, the literature search included only full, peer-reviewed, journal articles of original data. Review articles, editorials, letters, case studies, and abstracts were excluded. Studies were identified primarily through MEDLINE searches of the English language literature up to May 2001. Studies published between May 2001 and November 2002, that were identified through means other than the systematic literature searches, were included if appropriate.
Separate search strategies were developed for each topic. The text words or MeSH headings for all topics included kidney or kidney diseases, hemodialysis, peritoneal dialysis, or kidney transplant. The searches were limited to human studies, but included both adult and pediatric populations. Potential articles for retrieval were identified from printed abstracts and titles, based on study population, relevance to the topic, and article type. These were screened by clinicians on the Evidence Review Team. Overall, 10,363 abstracts were screened, 642 articles were retrieved, and 258 articles were subjected to structured review by members of the Work Group. Although systematic, manual searches were not conducted, members of the Work Group supplied a number of articles that were not located by the MEDLINE searches.
Work Group members used forms that were developed by the Evidence Review Team to extract information from each article that was reviewed. The Evidence Review Team used the information from these forms to construct the Evidence Tables. The Evidence Review Team then used the Evidence Tables to construct the Summary Tables that are included with the guidelines in this report. The Summary Tables describe the information in the Evidence Tables according to 4 study dimensions: size, applicability, results, and methodological quality.
The study (sample) size was used as a measure of the weight of the evidence. In general, large studies provide more precise estimates of prevalence and associations. Applicability (generalizability or external validity) addresses the issue of whether the study population is sufficiently broad so that the results can be generalized to the population of interest. The applicability of each article was determined using a three-level scale:
Methodological quality (internal validity) refers to the design, conduct, and reporting of the clinical study. Because studies with a variety of designs were evaluated, a broad classification system to rate the quality of individual studies was used:
There were no guidelines that were assigned an "A" level recommendation. The key guideline statements in this document were graded "B" or "C." Some would argue that no guideline statements should be made in the absence of evidence from randomized trials in patients with CKD (yielding level "A" recommendations). However, it was decided that when the strength of evidence for treatment efficacy was strong—based on trials in the general population—this evidence might be reasonably extrapolated to patients with CKD (Fig 3). Specifically, it was assumed that similar treatment efficacy as reported in the general population would be found if the trials were carried out in patients with CKD.
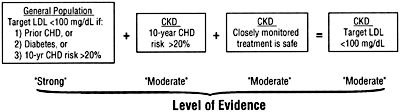
Fig 3. The chain of logic for evidence supporting the treatment of low-density lipoprotein cholesterol in patients with chronic kidney disease. See text for details. Abbreviations: LDL, low-density lipoprotein; CHD, coronary heart disease; CKD, chronic kidney disease.
This also assumes, of course, that treatment is safe and effective in ameliorating dyslipidemias in patients with CKD.
The principal results of large multicenter trials in the general population have generally been applicable to most, if not all, major subgroups of patients that have been examined (Fig 4). For example, the benefit of reducing LDL cholesterol extends to men and women3,46,47; old and middle-aged3,46,47; smokers and non-smokers3,47; hypertensive and non-hypertensive patients47; diabetics and nondiabetics48; and individuals with higher or lower LDL,3,47 higher or lower total cholesterol,3,47 higher or lower triglycerides,3,47 and higher or lower HDL.3,47,49,50 In other words, the results of lipid-lowering trials are usually generalizable to population subgroups.
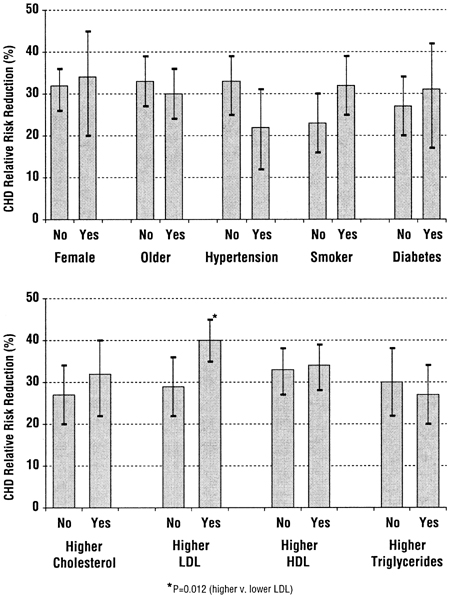
Fig 4. The relative coronary heart disease risk reduction in subgroups of patients from major lipid-lowering trials in the general population. The bars indicate the mean relative risk reduction (compared to a reference level of 0% reduction), with a higher number indicating a proportionally greater reduction in risk. The error bars represent 95% confidence intervals. There were at least 20,000 patients in each category (divided between "no" and "yes"). The only category where the risk reduction was statistically different was for baseline LDL (lower panel), where patients with a higher baseline LDL had a greater reduction in risk, although patients with lower and higher baseline cholesterol had significant risk reductions. Data are from Table II.2–3 of the Third Report of the National Cholesterol Education Program Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (www.nhlbi.nih.gov/guidelines/cholesterol/index.htm).
Therefore, it was reasonable to assume that the major findings from randomized trials in the general population are applicable to patients with CKD, until proven otherwise.
Nevertheless, there are reasonable doubts as to whether trial results from the general population can be extrapolated to all patients with CKD, and most major trials in the general population have excluded patients with elevated serum creatinine and Stage 5 CKD (Table 9). It is possible that, in some subpopulations of CKD patients, dyslipidemias may not play as large a role in the pathogenesis of CVD as they do in the general population. Therefore, it was concluded that additional studies are needed in patients with CKD (see IV. Research Recommendations). However, pending the results of these trials, the recommendations were based on the evidence from the general population.