Prior Evaluation and Therapy of Proposed Indications
Although there is evidence that L-carnitine administration may favorably affect the management of anemia (see below), it is essential that other potential issues be resolved before proceeding with L-carnitine therapy. For example, patients with persistent anemia despite the provision of erythropoietin should be thoroughly investigated for causes of erythropoietin resistence, including iron, folate, and vitamin B12 deficiency, chronic infection or inflammatory disease, advanced secondary hyperparathyroidism, and underdialysis. Efforts to correct these abnormalities (eg, iron supplementation, increase in dialysis dose) should be implemented before L-carnitine is used to treat anemia.
Intradialytic hypotension should be managed with meticulous attention to the dialysis procedure, and modification of the dialysis procedure should be considered. Prolongation of dialysis time, ultrafiltration profiling, sodium modeling, modification of dialysate sodium and calcium concentrations, and modification of dialysate temperature are among the changes in management that could be considered.
Causes of low cardiac output in ESRD patients should be thoroughly investigated. Pericarditis with tamponade is a life-threatening complication that can be diagnosed by careful physical examination and echocardiography. Left ventricular dysfunction should be managed with agents that provide afterload reduction (eg, angiotensin converting enzyme inhibitors) and have been shown to enhance survival in non-ESRD patients.268 Other agents proven effective in cardiomyopathy (eg, b -adrenergic antagonists) should also be considered.269 Symptoms of heart failure with normal or high cardiac output may be seen with conditions such as severe anemia, hyperthyroidism, and large or multiple arteriovenous shunts.
Malaise, asthenia, weakness, fatigue, and low exercise capacity are more complex entities, with few broadly effective therapies. Before considering L-carnitine for these conditions, underdialysis, abnormalities of thyroid function, primary neurologic diseases, sleep disturbances (including restless legs syndrome), depression, and other nutrient deficiencies should be considered and treated if present.
Specific Indications
For most potential indications, there was insufficient evidence from carefully conducted clinical trials to provide strong support for the use of L-carnitine. What follows below is a description of the evidence used by the Work Group to reach is conclusions. The level of detail provided roughly corresponds to the quantity and quality of available evidence.
Elevated serum triglycerides. The Work Group agreed that there was insufficient evidence to support or refute the use of L-carnitine for dialysis-associated hypertriglycedemia. Thirty-two studies were reviewed.270-301 Among 681 subjects, 55 maintenance hemodialysis patients served as controls. Thirty-one studies evaluated the serum triglycerides alone and one also reported on serum total cholesterol levels. L-carnitine treatment allocation was randomly assigned in 9 studies.270,272,274-277,279,280,301 L-carnitine was administered intravenously in 17 studies,270, 272, 275, 277, 280, 281, 284, 286, 287, 289-291, 294, 296, 297, 299, 301 orally in 13 studies,271,273,276,279,285,288,289,292,293,295,296,298, 300 and via the dialysate in 7 studies.274,278,282,283,287,292,298 Peritoneal dialysis patients were studied in one report.290 The average number of subjects was 21 per study (range, 6 to 97). The duration of L-carnitine treatment was heterogeneous, ranging from 1 week to 12 to 15 months, with the mean duration being 3 to 6 months. When administered intravenously, the dose of L-carnitine ranged from 1 m/kg body weight to 2 g at the end of each dialysis session, usually thrice weekly. Oral L-carnitine was administered in one to three daily doses, from 10 mg/kg body weight per day to 3 g per day. When L-carnitine was added into the dialysate, the final dialysate L-carnitine concentration was approximately 75 µmol/L or 150 µmol/L, corresponding to 2 g or 4 g of L-carnitine for each dialysis session, respectively.
There was no significant change in serum triglycerides in 23 of 32 studies. In a single study in which 3 g per day of oral L-carnitine were administered, there was a significant increase in serum triglycerides (+ 22%) over a 5-week time period. A decrease in serum triglycerides was observed in seven studies; in some of these, the significant decrease was observed in patient subgroups only, based on dialysate buffer,278 starting HDL concentrations,291 or the final dose of L-carnitine.280 The small sample sizes, heterogeneity in L-carnitine route of administration and dose, variable durations of study and methods of analysis, and the inclusion of patients with normal triglyceride levels in most studies make interpretation of these data difficult.
Cardiac function and arrhythmias. Cardiac and skeletal muscle myocyte metabolism is largely oxidative and dependent on free fatty acid delivery and mitochondrial transport. Moreover, the myocyte has one of the highest intracellular carnitine concentrations in the body. Experimental models of cardiomyopathy have been corrected with the administration of L-carnitine, and primary carnitine deficiency has been associated with left ventricular hypertrophy in animal models.
Cardiovascular disease accounts for approximately 50% of deaths in the ESRD population, and complications of left ventricular dysfunction and left ventricular hypertrophy lead to considerable morbidity.302 For these reasons, L-carnitine therapy has been explored as a treatment for cardiovascular disease in ESRD.
Two studies of L-carnitine treatment evaluated ejection fraction as an index of left ventricular function.303,304 Van Es et al303 showed a statistically significant increase in ejection fraction among 13 patients (mean, 48.6% versus 42.4%) after 3 months of L-carnitine therapy (1 g IV after each hemodialysis session). The patients had all undergone hemodialysis for greater than 1 year, using high-flux, bicarbonate dialysis, with hematocrit > 30% and with no change in hemodialysis frequency or time over the course of the study. The study was not randomized, and there was no concurrent control. Fagher et al304 conducted a 6-week, randomized placebo-controlled trial in 28 hemodialysis patients, who received either 2 g IV of L-carnitine or placebo after each hemodialysis session. There was no difference in ejection fraction comparing baseline and posttreatment values and no difference between L-carnitine and placebo groups. Furthermore, there was no difference in heart volumes. Although randomized and placebo-controlled, the study was short-term, and the patients included did not have evidence of myocardial dysfunction (mean ejection fraction, 62%).
As part of a multicenter, long-term (6 months), double-blind, placebo-controlled randomized clinical trial of 82 maintenance hemodialysis patients (see below),272 Holter monitoring was performed during a single dialysis period during the baseline (nontreatment) period, during the treatment period, and at the end of the treatment phase. Individual data were not available for review, but the authors noted that there were very few arrythmias at baseline in their study subjects, and no significant change in dialysis-associated arrhythmias was observed.
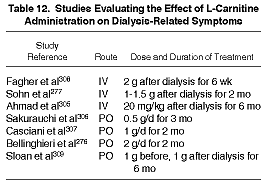
Malaise, asthenia, muscle cramps, weakness, and fatigue. Seven studies reported the effects of L-carnitine on either postdialysis fatigue,276, 305-308 muscle weakness,306 muscle cramps,277 or well-being.277,309 Only the study reported by Sloan et al309 included a well-accepted scale of health-related quality of life (the Medical Outcomes Study Short Form-36 instrument). The duration of treatment ranged from 2 to 6 months. The dose and route of delivery was widely variable, making comparison across studies difficult (Table 12).

In a double-blind, randomized, placebo-controlled study, Ahmad et al305 showed significant improvement over time in postdialysis asthenia in both L-carnitine-and placebo-treated patients; there was no significant difference in the response to treatment between the groups. However, it was only among the L-carnitine-treated patients that the authors found a significant reduction in intradialytic muscle cramps and hypotension. Sakurauchi et al306 reported that symptoms of fatigue were reduced in 14 of 21 patients, and muscle weakness improved in 14 of 24 patients (P < 0.05) after 3 months of L-carnitine treatment. There was no control group, and the methods of symptom assessment were neither adequately described nor validated. Sohn et al277 reported significant improvements in muscle cramps and sense of well-being comparing L-carnitine to placebo in 30 hemodialysis patients, although their methods of assessment were likewise not described. Casciani et al307 performed an 18-patient, nonrandomized cross-over study, and showed a significant improvement in asthenia after 2 months of L-carnitine administration, regardless of the order of drug administration. Bellinghieri et al276 evaluated muscle fatigability immediately postdialysis and during the interdialytic interval. They showed that postdialysis asthenia was markedly reduced as early as 15 days after commencing L-carnitine therapy, whereas intradialytic asthenia was only improved after 30 days of treatment. When L-carnitine was stopped, asthenia resumed within 15 to 30 days.276 By contrast, Fagher et al308 found no subjective improvement in fatigue in 14 patients treated with L-carnitine for 6 weeks.
Sloan et al309 provided oral L-carnitine (1 g before and 1 g after each dialysis treatment) to 101 maintenance hemodialysis patients and evaluated their health-related quality of life with the SF-36. In this study, oral L-carnitine had a perceived positive effect on the SF-36 general health (P < 0.02) and physical function (P < 0.03) subscales, although the effects were not sustained after 6 months of treatment.
In summary, although most studies of "subjective" symptoms suggest a beneficial effect of L-carnitine supplementation for maintenance dialysis patients, the Work Group concluded that the heterogeneity of study design, and the difficulty in measuring these and related symptoms in an unbiased manner render the available evidence in this area inconclusive. Nevertheless, several members of the Work Group felt that a short-term trial of L-carnitine was reasonable in selected patients with these symptoms who are unresponsive to other therapies, in light of its favorable side effect profile, lack of alternative effective therapies, and the findings from some studies of improvement in these symptoms with L-carnitine therapy.
Exercise capacity. Correction of anemia, hyperparathyroidism, and 1, 25-OH vitamin D3 deficiency and provision of adequate dialysis do not fully restore muscle function and exercise capacity in ESRD patients. Carnitine is abundant in skeletal muscle, and muscle carnitine content has been reported to decrease with dialysis vintage.277 Therefore, provision of L-carnitine might help to restore muscle mass and function. Five studies describing various aspects of physical activity were reviewed in detail. Physical activity was assessed by a patient activity score,310 exercise time, maximal oxygen consumption and mid arm muscle area,305 a measurement of maximum strength,308 exercise workload,308 and subjective muscle strength.280
The duration of treatment ranged from 1 to 6 months. L-carnitine was administered either IV at the end of each dialysis session, 2 g for 6 weeks307 or 6 months,311 20 mg/kg for 6 months,305 or PO 0.9 g/d for 2 months298 and 3 g/d for 30 days.280
Each study assessed physical activity in a different manner. Siami et al310 observed a trend (P = 0.07) toward improvement in subjective physical activity (on a scale from 1 [normal] to 5 [total incapacity]) after dosing L-carnitine, 2 g IV after dialysis for 6 months. Ahmad et al305 reported a significant increase in mid-arm muscle area (P = 0.05) in carnitine-treated patients and no change in placebo-treated patients. In the L-carnitine-treated patients, there was a significant increase in the maximal oxygen consumption (mean increase, 111 mL/min; P < 0.03) and a trend toward increased exercise time. Fagher et al308 observed an improvement in maximum muscular strength from baseline (P < 0.01) only in the group receiving L-carnitine 2 g IV after dialysis for 6 weeks, although there was no significant difference between treatment and placebo arms in this study. Mioli et al298 reported an increase in maximum work load after 45 days of oral L-carnitine administration that was sustained after 60 days of treatment (P < 0.05). Finally, Albertazzi311 reported a subjective improvement in physical activity (not quantified) in 10 patients receiving 3 g L-carnitine PO per day for 30 days and no change in 10 control subjects.
In summary, as with the more subjective symptoms of malaise, asthenia, muscle cramps, weakness and fatigue, there is inconclusive evidence regarding the role of L-carnitine supplementation on muscle function in ESRD. Although most of the published studies suggest a modest beneficial effect, relatively few studies are well-controlled, the methods of assessment are not validated, and assessment may be insensitive to important changes induced by a variety of therapies, including L-carnitine itself. The Work Group members were also concerned about the effect of publication bias on the available medical literature. In other words, it might be less likely for investigators to submit studies with a nil effect, and less likely that journal editors would publish such papers. The Work Group agreed that there was insufficient evidence to support the use of L-carnitine to enhance muscle strength or exercise capacity in patients on dialysis. However, the Work Group agreed that a short-term trial of L-carnitine (3 to 4 months) was reasonable in selected patients to enhance muscle strength and exercise capacity, in light of its favorable side effect profile, lack of alternative effective therapies, and benefits shown in several studies. More research is required in this area.
Anemia. It has been proposed that carnitine deficiency might reduce erythrocyte half-life, by adversely influencing the integrity of the erythrocyte membrane. Kooistra et al312 showed a relation between anemia and erythropoietin requirements and low serum free carnitine levels in dialysis patients. Despite the availability of recombinant erythropoietin and the more liberal use of intravenous iron dextran in recent years, a large proportion of maintenance dialysis patients continue to suffer from anemia or require large doses of erythropoietin to maintain blood hemoglobin concentrations within the recommended range. Epidemiologic studies have consistently shown a mortality advantage among patients with hematocrits in the 30% to 36% range, and the NKF-DOQI Work Group on Anemia Management recommended a target hematocrit of 33% to 36% based on the expert panels' detailed literature review.
Ten studies involving carnitine and anemia were reviewed in detail. Four studies272,314-316 (36 patients total) compared hemoglobin or hematocrit at baseline and after about 2 months of L-carnitine treatment (three studies using oral L-carnitine and one study using a combination of oral and intravenous L-carnitine). A fifth study292 was a nonrandomized trial in which 12 patients were treated with oral L-carnitine (1 g per day) and 11 patients were dialyzed against a bath supplemented with L-carnitine (concentration, ~ 100 µmol/L) for 6 months. Although three of the five studies showed significant improvement in blood hemoglobin or hematocrit, the Work Group discounted these studies due to flaws in design. A single cross-over study was performed.276 In only one of the two sequences was there a significant increase in hematocrit. There were 14 patients overall (7 in each sequence). The rather small sample size limited statistical power, and there was no consideration given to blood loss, iron status, or other clinical factors. It is noteworthy that in none of the six studies cited above were the hematologic effects of L-carnitine the primary outcome of interest.
Four randomized, placebo-controlled clinical trials272,275,315,316 were conducted in which the effect of L-carnitine on hemoglobin concentration or hematocrit was evaluated. In three of the four studies,272,314,316 treatment of anemia was the primary focus of the work. The total number of patients studied was 109. Nillson-Ehle et al275 treated 28 patients for 6 weeks with L-carnitine 2 g IV after each dialysis session. There were no significant differences in hemoglobin concentration in either group. No mention was made of serum levels or intake of iron, vitamins, or other factors known to affect management of this condition. In a randomized, placebo-controlled, double-blind trial, Labonia272 treated 13 patients with L-carnitine 1 g IV after each dialysis session for 6 months and compared the results with 11 patients given a placebo control. Inclusion criteria included a stable dialysis regimen, "normal" iron status, "usual" treatment with folic acid and vitamin B12, and the absence of "severe" hyperparathyroidism. In each patient, efforts were made to periodically reduce the dose of erythropoietin, but any reduction in the erythropoietin dose was maintained only if the hematocrit did not decrease. The target hematocrit was 28% to 33% throughout the study, and a protocol for erythropoietin dosing was established. There were defined, accepted criteria for the provision of iron supplements. The hematocrit remained stable in the L-carnitine-treated group, but dropped slightly (and significantly) in the placebo group (mean, 29.5% to 27.9%; P < 0.05). The erythropoietin dose requirements were reduced by 38% in the L-carnitine-treated patients and unchanged in the placebo-treated group. Roughly the same proportion of patients received iron during the course of the study, although the ferritin concentration (a marker of iron stores and of inflammation) was higher on average in the placebo group. There were no changes in endogenous erythropoietin or in erythrocyte osmotic fragility; thus, there was not a clear mechanism for what appeared to be a large clinical effect.
Trovato et al315 showed even more dramatic results in a placebo-controlled randomized study conducted before the availability of erythropoietin. In the control group, the mean hematocrit was 24.0% at baseline and dropped to 21.8% after 12 months. In the L-carnitine group, the mean hematocrit was 25.5% and increased to 37.4% after 12 months. All patients received folic acid, vitamin B12, and sodium ferrigluconate at the end of each dialysis session.
Finally, Caruso et al316 led a placebo-controlled randomized clinical trial in 31 hemodialysis patients, looking at erythropoietin dose and hematocrit. Patients received 1 g of L-carnitine IV after each dialysis session. The overall study results showed no significant effect of L-carnitine. When examining the subgroup of patients older than 65 years of age (n = 21), there were significant increases in hematocrit (mean, 32.8% versus 28.1%) and lowering of the erythropoietin dose (mean, 92.8 versus 141.3 U/kg) in the L-carnitine-treated patients compared with placebo-treated controls. It is worth noting that the Trovato et al315 and Caruso et al316 studies both employed per protocol analyses, compared with the more conventional "intent to treat" methods.
Some members of the Work Group felt that an empiric trial of oral or intravenous L-carnitine (≈1 g after dialysis) was reasonable in selected patients with anemia and/or very large erythropoietin requirements. A 4-month trial was considered to be of sufficient length to reliably assess the response to L-carnitine.