Treatments that lower urinary albumin excretion may slow progression of diabetic kidney disease (DKD) and improve clinical outcomes, even in the absence of hypertension. However, most people with diabetes and albuminuria have hypertension; management of hypertension in these patients is reviewed in Guideline 3.
1.1 Normotensive people with diabetes and macroalbuminuria should be treated with an ACE inhibitor or an ARB. (C)
1.2 Treatment with an ACE inhibitor or an ARB may be considered in normotensive people with diabetes and microalbuminuria. (C)
1.3 Albuminuria reduction may be considered a treatment target in DKD. (C)
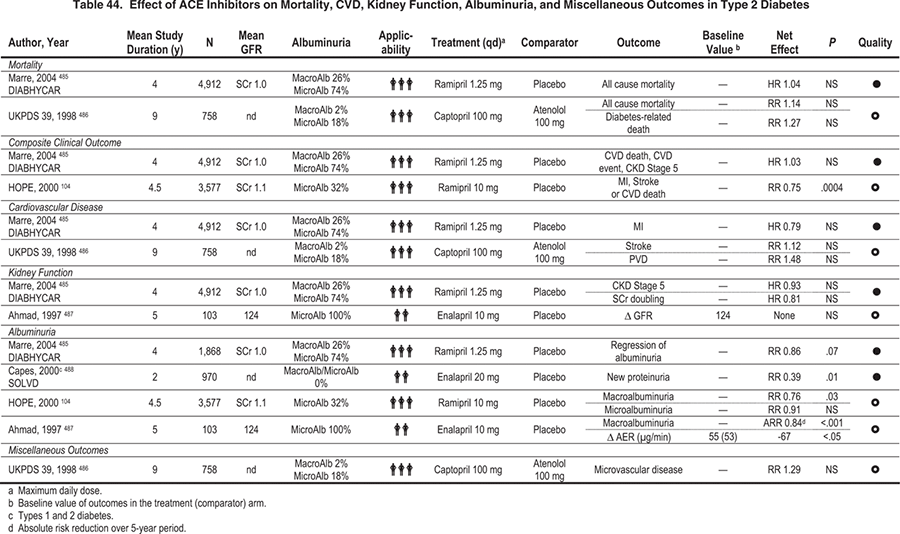
This CPR addresses the evidence for treatment of normotensive patients who have diabetes and elevated albuminuria with ACE inhibitors and ARBs. RAS inhibition effectively reduces albuminuria progression and improves clinical outcomes in hypertensive patients with DKD, but relatively few studies, particularly of antihypertensive agents, have specifically recruited normotensive people with diabetes and elevated albuminuria. Although there is a greater body of evidence that evaluates ACE inhibitors in type 1 diabetes and ARBs in type 2 diabetes (Table 44 to Table 46), the Work Group views their relative benefits as interchangeable for early and late stages of DKD. Accordingly, the Work Group assumes, as in Guideline 3, that a class effect exists across these agents, although several individual agents of each class have not been tested with clinical end points in kidney disease. This assumption is based on consistency among studies with agents of either class and it reflects the opinion of the Work Group. Nevertheless, the effectiveness of individual agents may differ.
The role of albuminuria change as a surrogate end point for clinical outcomes in the setting of DKD also is discussed. Albuminuria usually is present in DKD. Many studies in people with diabetes and microalbuminuria or macroalbuminuria have targeted stabilization or reduction in albuminuria levels as surrogate end points for progression of kidney disease. Studies evaluating interventions aimed at reducing albuminuria primarily used ACE inhibitors and ARBs.
Relationships between glomerular structural lesions and the presence or absence of microalbuminuria in diabetes are not straightforward. In addition, intrapatient variability in albuminuria measurements is large, and there is controversy about the standardization of the measurement itself. For all these reasons, the Work Group concluded that the evidence for using albuminuria as a surrogate marker for clinical outcomes was not sufficiently strong to merit a guideline statement. In turn, this conclusion influenced the Work Group's interpretation of the strength of the evidence for use of ACE inhibitors or ARBs in diabetic patients who are normotensive and have either macroalbuminuria or microalbuminuria. Therefore, the evidence ratings in CPR 1 were downgraded from those given for corresponding statements in the KDOQI™ Guidelines on Hypertension and Antihypertensive Agents in CKD.
Definitions
The definition of microalbuminuria is albumin-creatinine ratio (ACR) of 30 to 300 mg/g, and of macroalbuminuria, ACR greater than 300 mg/g (Guideline 1). The definition of hypertension is blood pressure of 130/80 mm Hg or greater (Guideline 3).
Normotensive people with diabetes and macroalbuminuria should receive an ACE inhibitor or an ARB. (Moderate/Weak)
In type 1 diabetes with macroalbuminuria, ACE inhibitors decrease albuminuria and reduce the risk of clinical outcomes regardless of the presence or absence of hypertension. A randomized controlled trial in people with type 1 diabetes and macroalbuminuria found that ACE inhibitors reduced the risk of the combined outcome of doubling of serum creatinine level, CKD stage 5, and death.168 A quarter of the participants were normotensive. There was no significant difference in the treatment effect between the normotensive and hypertensive individuals.

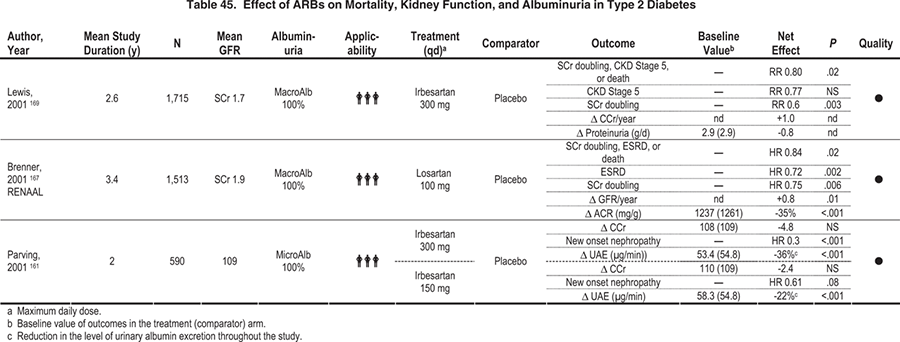

In type 2 diabetes with macroalbuminuria, ARB treatment reduces the risk of clinical outcomes. A 300-mg daily dose of irbesartan reduced proteinuria levels (significance not reported) and the risk of doubling of serum creatinine level compared with 10 mg daily of amlodipine or placebo in mostly hypertensive people with type 2 diabetes and nephropathy.169 In another study, losartan significantly reduced the ACR and the risks of CKD stage 5 and doubling of serum creatinine level compared with placebo.167 These 2 studies had very few participants with normal blood pressure.
Overall, patients with diabetes, macroalbuminuria, and normal blood pressure rarely were included in the available studies. Therefore, evidence for ACE-inhibitor or ARB treatment in these patients was considered moderate to weak. Nevertheless, based on this limited evidence, the Work Group recommends treatment with an ACE inhibitor or an ARB in this group of patients.
In normotensive people with diabetes and microalbuminuria, use of an ACE inhibitor or an ARB may be considered. (Weak)
Few studies have evaluated ACE inhibitors or ARBs for the treatment or prevention of microalbuminuria in normotensive people with type 1 diabetes. A meta-analysis of clinical trials in people with type 1 diabetes found that ACE inhibitors reduced both the level of albuminuria and progression from microalbuminuria to macroalbuminuria in normotensive subjects.203 In addition, a recent study (N = 73) found that only 8% of participants treated with 10 mg of enalapril daily compared with 31% of participants receiving a placebo developed microalbuminuria.204
Because most people with type 2 diabetes and albuminuria have hypertension, few studies have evaluated normotensive people with type 2 diabetes and microalbuminuria. One small study (N = 94) found that enalapril treatment reduced progression to macroalbuminuria by 30% (P < 0.001), with only 12% of patients in the treatment group progressing versus 42% in the placebo group.206 Similarly, another study (N = 62) found that enalapril significantly reduced albuminuria after 4 years of treatment, whereas participants randomly assigned to placebo experienced an increase in albuminuria.207 Another small study (N = 19) in normotensive people with either microalbuminuria or macroalbuminuria found that albuminuria increased over 2 years in the placebo group, but decreased significantly with perindopril treatment (P < 0.05).205 Similarly, ACE inhibitors may decrease albuminuria and reduce the risk of kidney and CVD outcomes. The Heart Outcomes Prevention Evaluation (HOPE) trial found that ramipril reduced the risk of the combined end point of myocardial infarction, stroke, or death due to CVD in people with type 2 diabetes. It also reduced progression to macroalbuminuria in subjects with microalbuminuria at baseline, but did not lower the risk of new cases of microalbuminuria.104
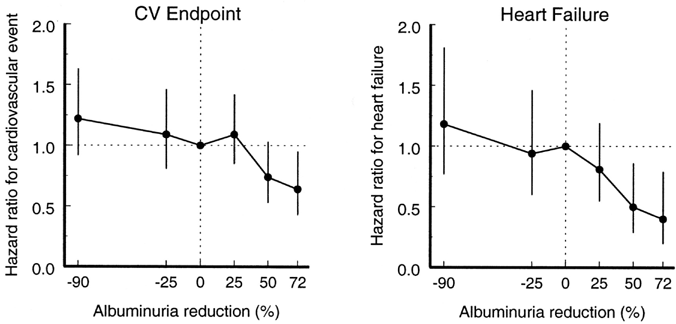
Figure 23. Hazard Ratios for CVD and Heart Failure End Points as a Function of Percent Change in 6-month Albuminuria in the RENAAL trial.
Relation is corrected for a
series of risk markers. Abbreviation:
CV, cardiovascular.
Reprinted with permission. 211
In the opinion of the Work Group, a change in albuminuria or transition between categories (normoalbuminuria, microalbuminuria, or macroalbuminuria) in normotensive people with diabetes is relatively weak evidence for change in status or prognosis of kidney disease. The rationale for this opinion is as follows. First, level of albuminuria or crossing an ACR threshold is not a clinical end point. Second, RAS inhibitors might mask the progression of DKD marked by albuminuria. In type 1 diabetes, withdrawal of ACE inhibition caused a rapid increase in albuminuria,208 and in type 2 diabetes, discontinuation of irbesartan in the IRMA-2 study prompted a rapid return to pretreatment levels of albuminuria in patients receiving the lower dose of irbesartan and a partial return to pretreatment levels in those receiving the higher dose of irbesartan.209 Third, few normotensive patients with diabetes and microalbuminuria have been enrolled in clinical trials of treatments for kidney disease. The demonstrated benefits of RAS inhibition for reducing and stabilizing albuminuria were noted, yet in the absence of studies with clinical end points, the Work Group found this evidence insufficient to justify a higher rating.
Albuminuria change may be an acceptable surrogate marker for clinical outcomes in DKD. (Weak/Opinion)
Studies testing the hypothesis that albuminuria reduction predicts improved prognosis in DKD have been performed only as secondary analyses of studies of ARB treatment in people with type 2 diabetes and macroalbuminuria.210-212 In these studies, level of albuminuria reduction was a marker of decreased risk of adverse outcomes. Observational analyses from the RENAAL trial found that the magnitude of albuminuria reduction predicted reduced risk of both CVD events and kidney end points (Fig 23 and Fig 24). 211,212 Similarly, an analysis from the IDNT found that degree of proteinuria reduction corresponded to decreased kidney end points (Fig 25). 210 These findings raise the hypothesis that albuminuria reduction per se has beneficial effects. However, an alternative possibility is that albuminuria reduction is a marker for patients with less severe kidney and vascular disease. A strategy of targeting treatment of albuminuria, in addition to blood pressure and other risk factors, has not been tested prospectively in patients with diabetes. Furthermore, to date, only these secondary analyses from the RENAAL trial and IDNT have directly correlated albuminuria/proteinuria reduction with clinical benefit.
New interventions to prevent or slow the progression of DKD are urgently needed. Interventions that reduce albuminuria or delay its increase may be promising as potential therapies for DKD. However, in the opinion of the Work Group, there currently is insufficient evidence to assume that lowering albuminuria levels will necessarily lead to improvements in clinical outcomes, such as progression to CKD stage 5, CVD events, or death. Conversely, the failure to reduce albuminuria does not preclude a beneficial clinical effect on DKD from a potential intervention. Therefore, to be considered efficacious, potential treatments for DKD must demonstrate benefits not only on albuminuria reduction, but also on such clinical end points as CKD stage 5, CVD events, or death.213
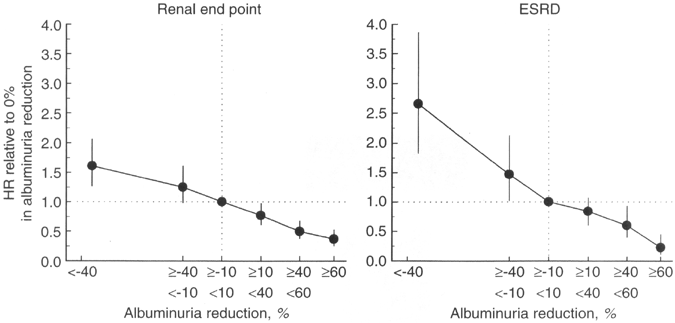
Figure 24. Hazard Ratios for Kidney End Points (Doubling of Serum Creatinine, CKD Stage 5, or Death) and CKD Stage 5 as a Function of Percent Change in 6-month Albuminuria in the RENAAL Trial.
Relation is corrected for a
series of risk markers. Abbreviation:
ESRD, endstage
renal disease. Reprinted
with permission.212
Most studies that assessed the efficacy of ACE inhibitors or ARBs in people with diabetes and albuminuria were conducted in people with hypertension or in a mix of subjects with and without hypertension. Therefore, there are not abundant data to direct therapy for normotensive people with diabetes who have microalbuminuria or macroalbuminuria. However, the consensus of the Work Group was that the benefits of ACE inhibitors and ARBs for reducing albuminuria and delaying kidney disease progression are likely to be similar among most people with diabetes and albuminuria, regardless of their blood pressure level.
In addition, in people with type 2 diabetes, microalbuminuria may represent early kidney injury or may be a manifestation of endothelial dysfunction and generalized vascular injury. The relative contribution of these 2 causes may vary in each patient. Given the uncertainty regarding the presence of kidney disease in subjects with microalbuminuria and the lack of clinical end points in trials of patients with diabetes and microalbuminuria, the Work Group's recommendations for use of ACE inhibitors and ARBs in normotensive people with diabetes and microalbuminuria are less strong than in those with macroalbuminuria.
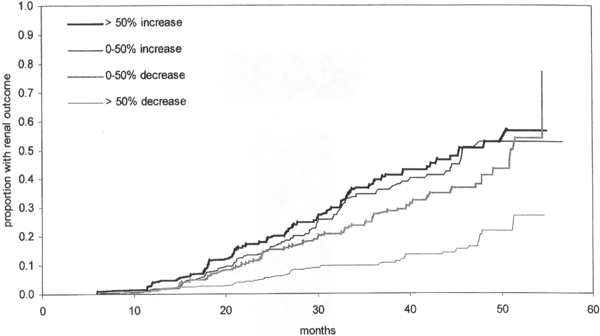
Figure 25. Kaplan-Meier Analysis of Kidney End Points (Doubling of Serum Creatinine [SCr], SCr Level > 6 mg/dL, or CKD Stage 5) by Level of Proteinuria Change in the First 12 Months of IDNT. Reprinted with permission.210
In normotensive people with diabetes and albuminuria, the target dose of ACE inhibitors or ARBs is unknown. In the absence of side effects or adverse events (eg, hyperkalemia), the Work Group recommends titration up to the maximum approved dose.
Placing people with microalbuminuria and diabetes on therapy with an ACE inhibitor or ARB may lead to less attention to glycemic control. The National Health and Nutrition Examination Survey (NHANES) 1999 to 2000 demonstrated that glycemic control has worsened in patients with diabetes and microalbuminuria, which may be caused by health care providers believing that RAS inhibition will reduce albuminuria and thus protect patients from clinical end points.484 The Work Group emphasizes the importance of glycemic control to prevent and treat albuminuria, as well as to reduce the overall risks of diabetes.