Data from RCTs suggested that the minimally acceptable small-solute clearance for PD is less than the prior recommended level of a weekly Kt/Vurea of 2.0. Furthermore, increasing evidence indicates the importance of RKF as opposed to peritoneal small-solute clearance with respect to predicting patient survival. Therefore, prior targets have been revised as indicated next.
2.1 For patients with RKF (considered to be significant when urine volume is > 100 mL/d):
- 2.1.1 The minimal “delivered” dose of total small-solute clearance should be a total (peritoneal and kidney) Kt/Vurea of at least 1.7 per week. (B)
- 2.1.2 Total solute clearance (residual kidney and peritoneal, in terms of Kt/Vurea) should be measured within the first month after initiating dialysis therapy and at least once every 4 months thereafter. (B)
- 2.1.3 If the patient has greater than 100 mL/d of residual kidney volume and residual kidney clearance is being considered as part of the patient's total weekly solute clearance goal, a 24-hour urine collection for urine volume and solute clearance determinations should be obtained at a minimum of every 2 months. (B)
2.2 For patients without RKF (considered insignificant when urine volume is ≤100 mL/d):
- 2.2.1 The minimal “delivered” dose of total small-solute clearance should be a peritoneal Kt/Vurea of at least 1.7 per week measured within the first month after starting dialysis therapy and at least once every 4 months thereafter. (B
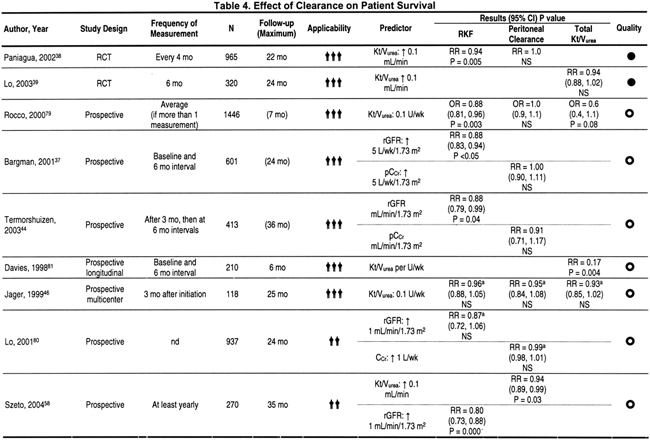
Previous studies suggested that improved survival on PD therapy was associated with higher total small-molecule clearances.36 Extrapolations from the Canada-United States (CANUSA) Study led to the prior guidelines of a total weekly Kt/Vurea of 2.0 and creatinine clearance (CCr) of 60 L/wk/1.73 m2 for CAPD patients. Higher targets were chosen for continuous cycling PD (CCPD) and patients on APD with no daytime dwell (dry day), and, in the absence of data, based on theoretical considerations. Reanalysis of the CANUSA Study showed that RKF, rather than peritoneal clearance, was associated with improved survival.37 Greater urine volume was a significant and important predictor of better survival, as well. Results of this reanalysis subsequently were supported by the Adequacy of PD in Mexico (ADEMEX) Study randomized trial of CAPD patients comparing 2 levels of PD prescription.38 The 2 groups of patients had identical survival, indicating no benefit on survival for greater small-molecule peritoneal clearance and confirming the benefit of RKF on survival. Further support was supplied by another randomized trial of CAPD patients from Hong Kong39 comparing 3 levels of total Kt/Vurea in patients with small degrees of RKF, with the lowest group randomized to a total Kt/Vurea of 1.5 to 1.7, with no difference in survival. Therefore, revision of the previous guidelines is needed.
Definitions
Total small-molecule clearance should be measured as Kt/Vurea and is based on a 24-hour collection of urine (kidney Kt/Vurea; if volume >100 mL/d) and a 24-hour collection of effluent for CAPD and APD, a sample of the effluent, and the total drained effluent volume (peritoneal Kt/Vurea; adding ultrafiltration with the infused dialysate volume). The term RKF is used to refer to estimated GFR, measured as the average of CCr and urea nitrogen clearance based on a 24-hour urine collection. Urine volume in 24 hours of 100 mL or less is considered to represent negligible RKF, although there are few data to indicate at what level kidney function becomes “negligible.” The term “delivered” peritoneal Kt/Vurea refers to the actual dose the patient is receiving based on measurement using the described method. This is distinct from an estimated peritoneal Kt/Vurea using a kinetic modeling program. “Delivered” Kt/Vurea assumes that the collection on the day the clearance is measured is representative of the patient's typical dialysis schedule and that the patient follows this same prescription every day.
For patients with RKF (considered to be significant when urine volume is >100 mL/d): the minimal “delivered”dose of total small-solute clearance should be a total (peritoneal and kidney) kt/vurea of at least 1.7 per week. (moderately strong evidence). Table 4 summarizes the effect of clearance on patient survival. In the ADEMEX Study, CAPD patients were randomized to continue on 4 exchanges using 2 L per exchange or to an increase in the prescription to provide a peritoneal clearance of 60 L/wk/1.73 m2 by either an increase in exchange volume or the addition of a nighttime exchange or both.38 The 2 groups had identical overall survival. Those with a mean total weekly Kt/Vurea of 2.27 had patient and technique survival equivalent to that of patients with a mean total Kt/Vurea of 1.80.38 Peritoneal small-molecule clearances bore no relationship to survival. In this study, body mass indices (BMIs) in the 2 groups were 25.3 and 25.8 kg/m2, and 42% to 45% of patients had diabetes, respectively. Patients were followed up for a minimum of 2 years, with 2-year survival rates of 68.3% and 69.3%, respectively. Approximately one half the patients had some RKF. The number of deaths in the 2 groups was identical, although causes of death varied slightly. In the ADEMEX Study, the group randomized to the lower prescription had slightly, but significantly, more deaths from congestive heart failure (CHF) and more deaths ascribed to uremia and hyperkalemia. This was balanced by an insignificantly higher number of deaths in the intervention group caused by coronary artery disease and peritonitis (although peritonitis rates were not higher). Deaths caused by CHF may have been greater in the control arm because ultrafiltration was less in this group (130 mL/d less, which represents 3.9 L/mo), likely because patients randomized to the higher prescription achieved this level through increased exchange volume (which is associated with higher ultrafiltration volumes) and, if necessary, a fifth exchange using a nighttime exchange device. Therefore, this difference in mortality caused by CHF may be due to differences in fluid removal.
QOL also was assessed in the ADEMEX Study. There were no significant differences between the 2 groups at any time for physical composite summary score, mental composite summary score, or kidney disease component summary.40 Therefore, neither survival nor QOL was benefited by greater small-molecule clearances.
Results of the ADEMEX Study are consistent with a subsequent randomized trial in Hong Kong comparing total Kt/Vurea values of 1.5 to 1.7, 1.7 to 2.0, and greater than 2.0 in CAPD patients.39 There were no differences in patient survival in the 3 groups. All patients at the start of the study had residual kidney Kt/Vurea of 1.0 or less, ensuring minimal RKF. Baseline residual GFRs (rGFRs) were 2.38, 2.48, and 2.64 mL/min/1.73 m2, respectively (representing kidney Kt/Vurea s of 0.44, 0.46, and 0.49 in the 3 groups, respectively; not a significant difference). Average BMI was 22 kg/m2, somewhat smaller than that of patients in the ADEMEX Study. The usual prescription was three 2-L exchanges per day, as opposed to four 2-L exchanges in the control arm of the ADEMEX Study. During the course of the 2-year study, PD prescription was adjusted up or down as RKF changed to stay within the randomized total Kt/Vurea category. By the end of the study, residual kidney Kt/Vurea was at or less than 0.1 in all 3 categories. Dialysis adequacy was assessed every 6 months. Results of these 2 important studies highlight the need to look at factors other than small-molecule clearance to improve survival in PD patients because peritoneal small-molecule clearance was not a predictor of survival, hospitalization, or nutritional state.
Observational studies support the findings of these 2 randomized trials, indicating that RKF (in those with RKF), rather than level of peritoneal small-molecule clearance, predicts survival, as well as QOL.41 In a large group of US PD patients (1,603 patients), age and serum albumin level were predictors of death, as was RKF; however, peritoneal clearance was not.42 Another study of 763 patients found that neither peritoneal Kt/Vurea nor peritoneal CCr was predictive of 1-year mortality.43 This population consisted of 53% CAPD and 34% CCPD patients; the rest were on both modalities during the 6-month study period or information was missing. In a longitudinal study of 412 adult PD patients (mean age, 52 years; 66.3% men, 15.3% with diabetic nephropathy), survival was predicted by GFR (RR, 0.88; 95% confidence interval [CI], 0.79 to 0.99; P = 0.039) and not peritoneal CCr. Comorbidity, albumin level at baseline, and age also were predictive of survival. Transport status was not a predictor of survival in this cohort. Kidney rGFR also was associated with multiple measures of better QOL, in contrast to peritoneal clearance, which was not associated with any component of QOL.44 In yet another study,45 transport status was not associated with survival, but survivors had significantly more residual function than those who did not survive (4.5 versus 2.8 mL/min/1.73 m2). Low initial RKF was associated with greater C-reactive protein (CRP) levels, indicating a relationship between inflammation and loss of RKF.
Observational studies suggest that volume status is closely linked to PD patient survival, as shown in Table 5. In a study from The Netherlands of 118 consecutive new PD patients examined in a prospective observational multicenter cohort study using Cox proportional hazards regression, systolic blood pressure (SBP; RR, 1.42 for every 10 mm Hg increase in blood pressure) was a predictor of survival, but peritoneal Kt/Vurea was not a predictor of survival, nor was kidney rGFR.46 Another study from The Netherlands examined poor outcomes (death or at least 2 of the following: prolonged hospitalization, serum albumin ≤3 g/dL, or malnutrition) in 189 patients and found that a model including comorbidity, serum albumin level, and physical and mental QOL was predictive of poor outcome, with a receiver operating characteristic (ROC) value of 0.84. A post hoc analysis excluding serum albumin level and QOL found that mean arterial blood pressure (MAP) was a strong predictor of poor outcome (MAP >107 mm Hg had an 8.6 times greater risk compared with MAP <107 mm Hg; P = 0.005), but only in PD patients, not HD patients.47 Similar results were found in an observational study from Turkey examining outcomes in 125 PD patients (who had survived ≥6 months on PD therapy), 92% of whom were on CAPD therapy. Comorbidity, serum creatinine level (likely a measure of nutrition), RKF, and hypertension (RR, 5.6; P = 0.001), but not peritoneal clearance, were predictors of survival.48 Another study showed that CRP level, RKF, and left ventricular mass index (LVMI) were all predictive of both all-cause mortality and cardiovascular death.49 An analysis of United States Renal Data System (USRDS) Wave 2 data regarding blood pressure in PD patients found that only low blood pressure (<111 mm Hg) was predictive of death, clearly a reflection of poor cardiac function because the finding was only present in those with a prior history of CHF (positive or suspected, 68% of total group).50 Of those with low blood pressure—in patients not administered antihypertensive medications (18% of total)—there were no associations between blood pressure and mortality. It is unclear whether this negative effect of low blood pressure was caused by a harmful effect on RKF, but this seems possible. All these studies suggest that close attention to volume status and blood pressure control are important in maximizing PD patients' chances of survival. Because of the emerging evidence about the importance of euvolemia, the Work Group has added Guideline 4.
Serum albumin level has been shown repeatedly to be a predictor of survival in dialysis patients. In a retrospective study from Turkey of 334 patients on CAPD therapy, survival was predicted by age, serum albumin level, cormorbid conditions (including peripheral vascular disease), and functional status, but not by Kt/Vurea.51 There are many causes of low albumin levels, including poor intake, chronic inflammation, chronic liver disease, volume overload, metabolic acidosis, and inadequate dialysis.52 There is little evidence that increasing small-molecule clearance improves serum albumin level. In neither the ADEMEX Study nor the randomized study from Hong Kong did higher peritoneal clearances lead to improvement in nutritional status.
Other maneuvers appear to be more successful in improving nutritional status. In a blinded randomized placebo-controlled trial of 60 CAPD patients with a total Kt/Vurea of 1.91 to 1.93 at the start of the study (and RKF of 1.78 to 1.91 mL/min), oral bicarbonate replacement was associated with an improvement in subjective global assessment (SGA) score and a decrease in anorexia.53 By the end of this 52-week study, total average Kt/Vurea values were 1.77 (treatment) and 1.78 (placebo). Three randomized trials examined the use of supplements to improve protein malnutrition.54-56 Protein powder (15 g [equivalent to 11 g of high biological value protein] administered twice daily) in CAPD patients with a total average Kt/Vurea of 1.7 to 1.8 was effective in improving SGA scores,55 whereas an oral liquid protein supplement was not effective, in large part because of poor tolerance.56 Likewise, a randomized trial of amino acid tablets in PD and HD patients found that the supplement improved serum albumin levels in HD patients, but not PD patients; adherence was poor in PD patients.54
Overhydration also is a cause of hypoalbuminemia in PD patients.57 Twenty-one patients (15 patients, CAPD; 6 patients, CCPD) had an increase in serum albumin level, decrease in blood pressure, and decrease in number of antihypertensive drugs after adjustment of the PD prescription to increase fluid removal. Therefore, the existing evidence suggests that if Kt/Vurea is 1.8 or greater and serum albumin level is low, attention should be directed toward correcting metabolic acidosis and fluid overload and consideration should be given to a palatable protein supplement. If Kt/Vurea is borderline (ie, <1.8), consideration should be given to increasing the dose of PD and to assessment of adherence with the prescription.
Surprisingly few data are available regarding the relationship between peritoneal small-molecule clearance and technique survival (Table 6). In the ADEMEX Study, overall withdrawal from the study and technique survival were similar for the 2 groups on differing PD prescriptions.38 Cause of withdrawal varied, with more patients in the control group withdrawing because of uremia (compared with none in the intervention group); however, by virtue of the study design, neither patients nor physicians could be blinded to the group. In the randomized trial from Hong Kong,39 withdrawal from the study was 6% because of inadequate dialysis and 8% because of inadequate ultrafiltration for the group randomized to a total Kt/Vurea of 1.5 to 1.7 compared with no patients withdrawn because of inadequate dialysis in the group randomized to a total Kt/Vurea of 1.7 or greater. In an observational study, higher peritoneal Kt/Vurea was an independent predictor of better technique survival in a group of patients with an average peritoneal Kt/Vurea of 1.59.58 In another observational study from the Netherlands Cooperative Study on the Adequacy of Dialysis (NECOSAD)44 combining patient and technique survival, there was no effect of peritoneal clearance on outcome. In 413 patients at 3 months on dialysis therapy, renal weekly Kt/Vurea was 0.82 and peritoneal weekly Kt/Vurea was 1.52. At 36 months of follow-up, 31 patients remained in the cohort, with essentially the same renal and peritoneal Kt/Vurea values. These results taken together suggest that setting the minimal total Kt/Vurea target at 1.7 should not have a negative impact on technique survival.
Measured total Kt/Vurea is not always the consistently delivered Kt/Vurea. Ultrafiltration may vary considerably from day to day, urine volume and GFR may fluctuate with volume status, and the patient may change the timing of the dialysis schedule or miss exchanges.59,60 Nonadherence with PD appears to vary by race (patients of Asian extraction are very adherent, for example), age (younger patients are more nonadherent than older), employment status (employed patients are more nonadherent than unemployed), and, possibly, country, indicating cultural influences.61,62 Therefore, in a patient who is not doing well on PD therapy, assessment of performance of the PD should be done.
Adherence can be evaluated by talking to patients and assessing inventory and use of supplies.63 In the ADEMEX Study, adherence was assessed by consumption of dialysis solutions; in the control group, 15.1 exchanges were missed per patient compared with 18.6 exchanges missed per patient in the intervention group.38 Because follow-up was a minimum of 2 years, this indicates that less than 1 exchange was missed per month, representing excellent adherence in these Mexican patients. Adherence has not been reported in the studies from Hong Kong, but it is possible that adherence with PD exchanges is significantly and importantly better than in the United States.
Close attention must be paid to the patient's commitment to fulfilling the prescription with the new lower targets. Perceived decreased control over future health, depression, and concern over restrictions that kidney disease imposes on daily life were all predictors of nonadherence.64 Few interventions have been done to decrease nonadherence; this is a critical area for future research.
To summarize, since the last guidelines were published, 2 randomized trials examining different levels of small-molecule clearance have been done in CAPD patients, showing no benefit of the higher small-molecule clearances on patient survival, nutritional status, hospitalization, or QOL. Emerging data suggest that the focus to improve survival in PD patients should be on preserving RKF, controlling volume overload (and thus blood pressure), treating metabolic acidosis, and perhaps use of protein supplements. Therefore, the minimal target is changed to a minimum Kt/Vurea of 1.7 per week, but careful attention must be paid to adherence to the prescription. The Work Group wishes to emphasize that this minimal target should not be interpreted as an average value for a program, but that each patient should have a total Kt/Vurea at 1.7 or higher.
For patients with RKF, total solute clearance (residual kidney and peritoneal, in terms of weekly Kt/Vurea) should be measured within the first month after initiating dialysis therapy and at least once every 4 months thereafter. If the patient has greater than 100 mL/d of residual kidney volume and residual kidney clearance is being considered as part of the patient's total weekly solute clearance goal, a 24-hour urine collection for urine volume and solute clearance determinations should be obtained at a minimum of every 2 months. In the CANUSA Study, RKF and peritoneal clearances were measured at baseline and every 6 months.65 During this 2-year study, kidney CCr decreased from 38.8 to 14.3 L/wk/1.73 m2, a rate of decrease of 1 L/wk/1.73 m2/mo, or 0.1 mL/min/mo. The change in total small-molecule clearance was caused almost entirely by the gradual decrease in RKF because few changes were made in PD prescription. Therefore, if small-molecule clearance is dependent on RKF and the PD prescription, close monitoring of kidney function appears warranted.
In the randomized trial from Hong Kong, patients within each category had the prescription adjusted (either an increase or decrease) so that total Kt/Vurea was within the target of each group (1.5 to 1.7, 1.7 to 2.0, and >2.0).39 Entry criteria required an initial kidney Kt/Vurea less than 1.0; average kidney Kt/Vurea values at the start were 0.44, 0.46, and 0.49 (not significantly different) for the 3 groups, and this number was added to the peritoneal clearance. The PD prescription was, in turn, adjusted to reach the total target. The first adequacy assessment was done at 4 to 8 weeks after starting CAPD therapy, and a reassessment was done 4 to 6 weeks after adjusting the prescription. From that point on, clearances were obtained every 6 months. During the course of the study, there was a steady decrease in RKF in all 3 groups, such that by 37 months, average kidney Kt/Vurea was less than 0.1 in all 3 groups.
There is considerable variability in the rate of RKF loss in PD patients.66 Therefore, to prevent patients from falling below the minimum total Kt/Vurea target of 1.7, when RKF is included in the determination, it appears prudent to obtain a 24-hour urine measurement every 2 months. Because peritoneal Kt/Vurea does not change much over time unless the prescription changes, every 4 months is believed to be adequate for measurement of peritoneal Kt/Vurea unless a change in RKF is noted.
For patients without RKF (considered to be insignificant for urine volume ≤100 mL/d), the minimal “delivered” dose of total small-solute clearance should be a peritoneal Kt/Vurea of at least 1.7 per week measured within the first month after starting dialysis therapy and at least once every 4 months thereafter. There are no RCTs of small-molecule clearance doses that examine outcome in only anuric patients. However, in the ADEMEX Study, anuric patients (defined as GFR <1 mL/min and constituting 56% of the control group and 54% of the intervention group) were analyzed separately. There was no survival benefit to increased small-molecule clearance in anuric patients. Although values for peritoneal Kt/Vurea are not given for this subset, for all patients in the study, peritoneal Kt/Vurea values were 1.58 and 1.59 at baseline and 1.62 and 2.13 averaged across the study duration, respectively38 The control CAPD prescription was 2 L times 4 exchanges. These results suggest that peritoneal Kt/Vurea of 1.62 in anuric CAPD patients results in the same survival as for those with Kt/Vurea of 2.1.
Most studies examining the relationship of Kt/Vurea to outcome in anuric PD patients come from Hong Kong. A descriptive study of a cohort of 140 anuric Chinese CAPD patients showed a relationship between small-molecule clearance and patient survival.67 In this study, mean weekly Kt/Vurea was 1.72 (confidence limits, 1.1 to 2.23, indicating that a number of patients had low peritoneal Kt/Vurea). The 2-year survival rate was 68.8%, similar to the ADEMEX Study. Peritoneal Kt/Vurea was an independent predictor of survival at this lower range of Kt/Vurea.67 The usual prescription in these smaller patients (BMI, 23.4 kg/m2 on average) was 3 times 2 L/d, with increased volume per exchange prescribed only with poor ultrafiltration despite use of increased dextrose dialysate.
Another retrospective analysis of Chinese CAPD patients (n = 168) compared 49 anuric patients (GFR, 0.7 and 0.05 mL/min/1.73 m2) with an average Kt/Vurea of 1.93 ± 0.19 with 71 patients with Kt/Vurea of 1.38 ± 0.22 and found that 1-year survival rates were 91.8% and 87.3%; hospitalization and technique survival were not different, but normalized protein equivalent of total nitrogen appearance (nPNA) decreased a bit more in the group with the lower Kt/Vurea, although this difference was insignificant (delta, 0.02 versus 0.04 g/kg/d decrease). Of note, patients with the higher Kt/Vurea were on an average exchange volume of 8 L/d, whereas those with the lower clearance were on 6 L/d.68 Anuric CAPD patients not only have greater overall mortality than nonanuric patients, the cause of the increase can be attributed to sudden cardiac death.69 These data suggest that Kt/Vurea of 1.7 (>1 SD greater than the mean for the group with the lower Kt/Vurea) should be sufficient in anuric patients. Close attention must be paid to cardiac risk factors to prevent sudden death in these patients.
Another observational study from Hong Kong suggests some benefit of increasing dose of dialysis, but with a plateau effect. The study examined outcome and risk factors for death in 150 anuric PD patients (defined as 24-hour urine <100 mL).70 After anuria developed (at a mean time on PD therapy of 44.1 months, with subsequent follow-up with anuria of 50.0 months), patients with a baseline peritoneal Kt/Vurea less than 1.67 were more likely to die than those with peritoneal clearance greater than this (RR, 1.985; P = 0.01). Baseline Kt/Vurea at the start of anuria was not predictive of mortality with Cox proportional hazard survival analysis (RR, 0.919 for every 0.1 increase, 0.833 to 1.014; P = 0.094). Survival was identical for those with Kt/Vureagreater than or less than 1.80 (P = 0.98), but in the subpopulation with Kt/Vurea less than 1.8 at baseline anuria, a subsequent Kt/Vurea greater than 1.76 resulted in better survival than for those with a clearance less than this (P = 0.033). In this observational study, PD prescription was changed to increase Kt/Vurea after anuria occurred. Women, in particular, were at increased risk for death with a Kt/Vurea less than 1.67.
An observational study compared CAPD patients with total Kt/Vureaof 2.03 because of significant RKF with those with total Kt/Vurea of 1.93 and very little RKF (RKF = 0.30 mL/min/1.73 m2) with a third group with very little RKF and total Kt/Vurea of 1.38 (RKF = 0.29 mL/min/1.73 m2).68 Patients in the 2 groups with equivalent Kt/Vurea (66% and 96% because of peritoneal rather than RKF, respectively) had equivalent survival and nutritional status. The group with the lower Kt/Vurea (1.38; 96% from peritoneal and virtually no RKF) had equivalent survival, hospitalization, and technique survival, but baseline normalized protein catabolic rate (nPCR; grams per kilogram per day) and percentage of lean body mass were worse for those patients compared with both other groups.
A single dialysis center observational cohort study of 270 CAPD patients followed up to 6 years with average total Kt/Vurea of 1.78 ± 0.4 and peritoneal Kt/Vurea of 1.59 ± 0.37 (0.82 to 2.33) showed in prevalent patients only (as opposed to incident) that an increase of 0.1 in peritoneal Kt/Vurea was associated with 9% better survival (RR, 0.91; 0.85 to 0.98). Because prevalent patients would have much lower (if any) RKF, this study supports the hypothesis that only at low levels of small-molecule clearance does peritoneal clearance have an impact on survival.58
The European Automated Peritoneal Dialysis Outcome Study (EAPOS) was a prospective multicenter study of outcomes in anuric APD patients (n = 177).71 One half the patients were using icodextrin for the long exchange. Time-averaged analyses showed that age, SGA grade C, and diabetic status predicted patient survival. Time-averaged CCr and baseline solute transport status had no effect on patient or technique survival. The range of CCr (liters per week per 1.73 m2) was 55.2 to 76.6 in this study.71 At baseline, 12% of patients had a body surface area (BSA) greater than 2.0 m2, and mean CCr ranged from 46 L/wk/1.73 m2 for low-average transporters to 75 L/wk/1.73 m2 for high transporters. EAPOS results suggest that large anuric patients, including those with low-average transport status, can be maintained successfully on APD therapy.
The NECOSAD Study Group, a prospective multicenter cohort study of new adult dialysis patients, recently released results of a study examining the relationship between small-solute clearances in anuric PD patients (n = 130).72 At the point of anuria, patients had been on PD therapy (primarily CAPD) for an average of 13 months and peritoneal weekly Kt/Vurea was 1.8. Mean BMI was 24.8 kg/m2. Anuria in this study was defined as urine output less than 200 mL/d. When Kt/Vurea was analyzed as a time-dependent continuous variable correcting for age, Davies score, SGA, time on dialysis therapy, serum albumin level, and hemoglobin concentration, there was no relationship with survival. When Kt/Vurea was analyzed as a dichotomous value (<1.7 versus ≥1.7), there was no relationship with survival. Only when Kt/Vurea was analyzed as a dichotomous value (<1.5 versus ≥1.5) could a relationship with survival be seen (RR, 3.28; 95% CI, 1.25 to 8.60; P = 0.02). These results are consistent with those of the 2 RCTs previously discussed, which did not show a survival benefit of increased small-molecule clearance in PD patients.
To summarize, although data are limited, peritoneal weekly Kt/Vurea of 1.7 in anuric patients on CAPD therapy appears to be an adequate minimal target. Randomized trials assessing different levels of peritoneal Kt/Vurea in anuric patients are needed. Randomized trials to assess different targets in APD patients also are needed.
In patients who are anuric, the dose of total small-solute clearance should be measured within the first month after starting dialysis therapy and at least once every 4 months thereafter. A retrospective analysis examined clearances in 115 anuric patients (89 patients, CAPD; 26 patients, APD).73 Anuria was defined as urine output less than 100 mL/d or kidney CCr less than 1 mL/min. Clearance studies were obtained every 3 months. This permitted adjustment in the prescription in an attempt to meet KDOQI targets, which were Kt/Vurea of 2 or greater for CAPD patients and 2.2 or greater for APD patients. Fifty-six percent of patients had a change in prescription after the onset of anuria, and 25% of these patients had an additional change based on the collections. Therefore, frequent measurement of peritoneal Kt/Vurea in anuric patients permits timely adjustment of the prescription.
A study assigned 100 anuric CAPD patients in nonrandom fashion to either an increase (n = 50) or no change in prescription (n = 50) and repeated the clearance at 6 months.74 Patients with an increase in prescription (peritoneal Kt/Vurea increased from 1.58 to 1.91) had an improvement in daily ultrafiltration, increase in nPNA (from 0.91 to 1.10 g/kg), and increase in percentage of lean body mass (all significant) at 6 months, whereas there were no changes in any parameters in control patients (Kt/Vurea = 1.66 at month 0 and 1.69 after 6 months). This nonrandomized trial suggests that patients with Kt/Vurea less than 1.7 may benefit from intervention. Therefore, frequent collections appear warranted.
There are only 2 randomized trials of dialysis dose in PD patients. The study designs were different in that the ADEMEX Study targeted a higher level of peritoneal clearance (not quite achieved), whereas the Hong Kong trial targeted 3 levels of total Kt/Vurea, combining kidney and peritoneal clearance to achieve this and adjusting the PD prescription to stay within the indicated goal. Each study had a homogeneous ethnic population (Mexican and Chinese, respectively). Therefore, the ability to apply these results to different ethnic groups and more culturally heterogeneous populations is limited and is the reason that the evidence is listed as moderate, rather than strong. Of particular concern is the variability in adherence to home prescription in other cultures in which adherence was shown to be problematic in some patients.
Data are limited in anuric patients. There are no randomized trials examining different prescribed and delivered doses of peritoneal small-molecule clearance in completely anuric patients. Slightly more than one half the patients in the ADEMEX Study were essentially anuric and a subanalysis was performed, but the study was not specifically designed to study this population.
There is even less information on levels of prescribed dose for CCPD, and even more limited on APD with dry days. There are no randomized trials comparing different doses on CCPD therapy or comparing CCPD with APD with a dry day. Of particular interest are patients who start PD with APD with a dry day and subsequently have a day exchange, and then 2 day exchanges added, a form of incremental dialysis. Theoretically, this might protect the peritoneal membrane from 24-hour glucose exposure, but middle-molecule clearance would be restricted with such an approach if applied early in the course of PD. In view of the very limited data about APD clearances and outcomes, no guidelines are possible for small-molecule clearance on APD therapy.
There are no randomized trials examining middle-molecule clearances in PD patients. Because emerging data suggest a plateau effect of small-molecule clearances on outcome in both PD and HD patients, attention should be turned to other uremic toxins. For example, there are no randomized long-term trials examining risk for neuropathy with these relatively low levels of PD prescription because this may appear after several years and the present studies examine 2 to 3 years. Longer term trials are necessary.
The prescribed dose of PD, as is true of HD, is not invariably the delivered dose. Patients adjust the timing of exchanges, eliminate exchanges, and change the dextrose of the dialysis solution, resulting in variations in ultrafiltration that, in turn, affect small-molecule clearance. Patients are responsible for their dialysis delivery, yet depression is common in PD patients, which may impact on adherence.75,76 Close attention must be paid to the patient's ability to perform (mentally and physically) his or her dialysis.
Furthermore, RKF does not remain stable. It is affected by volume status and tends to decrease over time. Therefore, if including residual kidney clearance as part of total Kt/Vurea, the measured dose of Kt/Vurea may not precisely reflect the delivered dose of Kt/Vurea, which will be less in some cases. This means that the clinician should err on the side of a higher prescribed dose when possible.
Implementation of the goal of euvolemia in PD patients involves close monitoring of urine volume, ultrafiltration, and physical examination, including blood pressure. Both home records and in-center measurements are needed. Frequent contact with the patient to supervise the use of the appropriate dialysis dextrose solution is necessary. The use of loop diuretics may be indicated to increase urine volume as appropriate (discussed later). “Negative” ultrafiltration with the long exchange should be avoided by adjusting the prescription and dialysate dextrose solution.
In 1999, the Canadian Guidelines for Adequacy and Nutrition in PD were published.77 For CAPD and APD, the minimum weekly Kt/Vurea clearance target was set at 2.0. CCr targets were 60 L/wk in high and high-average peritoneal transporters and 50 L/wk in low and low-average peritoneal transporters. This was given as an opinion. Clearance values for Kt/Vurea of 1.7/wk and CCr of 50 L/wk were considered almost always unacceptable. The recommendation was to perform a collection within 6 to 8 weeks of starting PD therapy and then, ideally, every 6 months, unless the prescription was changed or clinical status changed unexpectedly. A cautionary note was to be aware of the potential for noncompliance with exchanges. Clinic visits were considered to be adequate every 2 to 3 months unless the patient was not doing well. It was recommended to perform a peritoneal equilibration test (PET) within 6 weeks of initiating PD therapy. Special attention should be paid to state of hydration, serum albumin level, and nutritional status in high transporters. The importance of controlling volume overload and hypertension was emphasized.
The draft document from November 21, 2003, of the Canadian Society of Nephrology Clinical Practice Guidelines on PD Adequacy, not yet finalized, indicates that the term “adequacy” must be defined more broadly, rather than limited to only small-molecule clearances. The authors suggest that adequate dialysis includes attention to volume status and nutrition and cardiovascular risk reduction. Focus on preservation of RKF also is necessary. The Canadian draft guidelines contain 6 sections. The first indicates that peritoneal Kt/Vurea should be maintained at a minimum of 1.7 per week in both CAPD and APD patients when kidney rGFR is less than 4 mL/min. In patients with GFR greater than 4 mL/min, peritoneal Kt/Vurea may be maintained at 1.0 to 1.7. If the patient appears uremic, the peritoneal prescription should be increased. The draft guidelines emphasize the importance of considering lifestyle issues of the patient and caretakers, if any, and the effect of cumulative exposure to glucose. If peritoneal clearance is less than 1.7/wk because of dependence on RKF, the recommendation is to measure GFR every 2 months. Peritoneal Kt/Vurea can be measured every 6 months unless there is an unexpected change in the patient's condition. One section in the draft document is devoted to volume status and blood pressure. Emphasis is placed on appropriate prescription (in particular, a reasonable dwell time of at least 2 hours to permit sodium removal) and use of icodextrin and diuretics, as appropriate. If blood pressure is greater than 130/80 mm Hg, the investigators recommend achieving euvolemia and, if not effective, adding an antihypertensive, giving preference to an angiotensin-converting enzyme (ACE) inhibitor.
The Australian PD guidelines are published online (www.cari.org.au; last accessed 2/14/2006).77A As evaluation of adequacy, the guidelines recommend including clinical assessment of well-being, physical measurements, small-solute clearance, fluid removal, and the impact of treatment on the individual's life. Clearances alone (either greater or less than the target) should not be interpreted as representing adequate or inadequate dialysis. For CAPD and APD, weekly Kt/Vurea is recommended as 2.0, with a minimum of 1.7/wk. Minimum CCr target is given as 60 L/wk in high and high-average transporters and 50 L/wk in low-average and low peritoneal transporters. Kt/Vurea less than 1.7 and corrected CCr of 50 L/wk should be considered unacceptable for a patient with a BMI of 20 to 26 kg/m2. Emphasis is placed on not using small-solute clearance alone, but interpreting results together with clinical and laboratory assessments, including hydration status, blood pressure and lipid control, bone disease, anemia, and nutrition.
The Renal Association (UK) Guidelines, published in August 2002, recommend a total weekly CCr, combining dialysis and RKF, of 50 L/wk/1.73 m2 and/or weekly dialysis Kt/Vurea of 1.7 or greater.77B These should be measured by 6 to 8 weeks after the start of dialysis therapy and repeated at least annually, more often if RKF is rapidly decreasing. The suggestion is made that high or high-average transporters and APD patients may need higher targets.
The European Best Practice Guidelines for PD were initiated in 1999 and published in 2005.78 The minimum peritoneal target for Kt/Vurea in anuric patients is 1.7, identical to that in the present guidelines, but in addition, the guidelines recommend net ultrafiltration in anuric patients of 1.0 L per day. This guideline is believed to be evidence based (level B). No specific targets are provided for those with RKF other than to note that RKF can compensate when these peritoneal targets are not achieved. A higher Kt/Vurea target for APD was not recommended, with the rationale of the rapid diffusion of urea during short cycles, such as occurs with the cycler at night. However, the guidelines recommend achieving a minimum CCr of 45 L/wk/1.73 m2, as well as a minimum Kt/Vurea of 1.7 in patients on the cycler (evidence level C).