Prospective randomized trials of dialysis adequacy and many observational studies have confirmed a strong association between the presence of RKF and reduction of mortality in patients on PD therapy.
3.1 It is important to monitor and preserve RKF. (A)
3.2 In the patient with RKF who needs antihypertensive medication, preference should be given to the use of ACE inhibitors or angiotensin receptor blockers (ARBs). (A)
3.3 In the normotensive patient with RKF, consideration should be given to the use of ACE inhibitors or ARBs for kidney protection. (B)
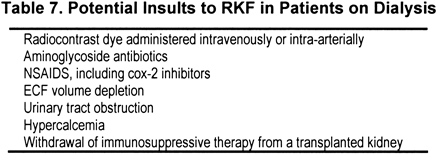
3.4 Insults to RKF (see Table 7) in patients with CKD also should be considered insults to RKF in PD patients and should be avoided when possible. (B)
Studies of the adequacy of PD, measured by small-solute clearance (Kt/Vurea and CCr), have shown that in the presence of RKF, outcome is driven by the kidney component only. In studies in which both the kidney and peritoneal contribution to small-solute clearance are measured, RR for mortality is related inversely to only the kidney component.37,41,42,46,79 There is no significant association between peritoneal small-solute clearance and outcome. It is only in studies of anuric patients that peritoneal clearance parameters are associated with outcome,67,73 and even then, peritoneal ultrafiltration may be more important than peritoneal small-solute clearance.71 The mechanism(s) involved in the robust association between RKF and reduction in mortality are purely speculative.
One possible benefit of preserved kidney function may be the kidney excretion of salt and water, which helps maintain euvolemia. In the reanalysis of the CANUSA Study, residual urine volume was more important than residual kidney small-solute clearance in predicting outcome.37 Furthermore, other studies showed that preserved kidney function is associated with both better blood pressure control and maintenance of more normal cardiac geometry.48,87,88
Another explanation for the benefit of RKF is that ongoing kidney clearance of uremic solutes contributes in a more significant way to reduction in mortality than that afforded by peritoneal clearance. Why kidney Kt/Vurea or CCr should reduce mortality while peritoneal Kt/Vurea or CCr does not very likely lies in other solutes cleared by the kidneys and perhaps less well-cleared by the peritoneal membrane. In other words, kidney small-solute clearance parameters serve as a marker of ongoing kidney function, but the benefit of the function is in the removal of other unmeasured uremic toxins.
Another possibility is that the association of preserved kidney function and better outcome is not the direct result of any excretory function of the kidney (eg, salt, water, small or large solutes). It may be that intrinsically “healthier” or relatively “uninflamed” patients may have a slower decrease in RKF. Studies have reported comorbid disease to be associated with faster decrease in RKF in patients on dialysis therapy89; thus, the absence of comorbid disease would be associated with relative preservation of kidney function. Therefore, the better outcome in dialysis patients with more preserved kidney function may be a marker of the relative absence of comorbid disease in these patients, rather than a particular life-prolonging function of the kidneys themselves.
Large population studies showing an association between decrease in kidney function and adverse cardiac events have led to the “cardiorenal” hypothesis. This hypothesis states that loss of kidney function increases the chance of cardiac-associated mortality in a manner that is not readily explained by traditional cardiac risk factors, such as lipid disorders. Healthy kidneys are associated with an absence of inflammation, and the increasing incidence of cardiac events with even minor decrements in kidney function may reflect the loss of this antiinflammatory function. This has led to the observation that patients with decreasing kidney function are more likely to die of cardiac causes than to reach CKD stage 5.90 However, in those who reach the need for dialysis, the association of further decrease in RKF with adverse events may simply reflect the same cardiorenal process, albeit now at the dialytic end of the spectrum of kidney function.
In the absence of certainty about which, if any, aspect of kidney function is associated with the improved outcome, it seems reasonable to try to preserve kidney function for as long as possible in patients on dialysis therapy.
Definitions
RKF represents the function of the native kidneys or the in situ kidney allograft. GFR is estimated by the numerical average of the 24-hour CCr and urea nitrogen clearance. Urine volume is the volume of urine produced in a 24-hour collection period. Anuric patients are those for whom 24-hour urine volume is considered insignificant, arbitrarily chosen as 100 mL/d or less. However, as mentioned in Guideline 2, it is unclear at what volume or GFR the contribution of RKF is considered negligible and the patient is functionally anuric.
It is important to monitor and preserve RKF. Although the explanation for the association of preserved RKF with survival is not known (see Background), the association is so robust in studies from around the world that preservation of this function should be a major objective in the management of dialysis patients. Furthermore, although the benefit of increasing dialytic (HD or PD) clearance appears to plateau eventually,38,91 no such asymptotic function holds for RKF. The ultimate extrapolation would be to normal kidney function, and survival in this group is many fold greater than in those with no kidney function.92
It is reasonable to assume that interventions that slow the decrease in kidney function in patients with CKD also will slow the decrease in RKF in patients on dialysis therapy. Furthermore, agents or events that are nephrotoxic in general can be assumed to be nephrotoxic to RKF. There are different levels of evidence to support these assumptions.
In the patient with RKF who needs antihypertensive medication, preference should be given to the use of ACE inhibitors or ARBs. The last 2 decades have seen a plethora of studies showing that control of blood pressure, particularly by the use of ACE inhibitors and ARBs, is associated with a decrease in the slope of decline in kidney function in patients with kidney disorders, particularly those with diabetic kidney disease or glomerulonephritis.93-97 In many studies, the salutary effect of ACE inhibitors and especially ARB agents was seen with little or no change in blood pressure control. Again, can the assumption be made that interventions that slow the decrease in GFR in patients with CKD also work in dialysis patients?
A retrospective study of more than 200 PD patients found that patients not administered antihypertensive drugs had a faster decrease in their kidney function.89 In analysis of data from the USRDS, use of an ACE inhibitor or calcium channel blocker was associated with decreased loss of RKF, defined as urine volume greater than 200 mL/d.98
These observations led to 2 RCTs that examined the effect of ACE inhibition and angiotensin receptor blockade on RKF in PD patients. In the first study, 60 PD patients were randomized to receive 5 mg of ramipril or no treatment. Other antihypertensive agents could be used. At the end of 1 year, the subgroup administered the ACE inhibitor had just less than 1 mL/min greater GFR compared with those not administered the drug.99 A similar study, albeit in even fewer patients, showed that 40 to 80 mg/d of valsartan was associated with a slower decrease in GFR and urine volume at 24 months, without a difference in blood pressure.100 Although the number of patients in each study was small, there is consistency between the 2 studies and a believable physiological underpinning to the findings. For this reason, the use of these agents is recommended when antihypertensive therapy is indicated for PD patients.
In the normotensive patient with RKF, consideration should be given to the use of ACE inhibitors or ARBs for kidney protection. It is not clear how much of the renoprotective effect of ACE inhibitors or ARBs is related to their antihypertensive effect versus other mechanisms.
Studies of nondialysis populations suggested that the renoprotective effect is, in part, independent of effects on blood pressure. Therefore, these agents often are used in patients with CKD, especially those with glomerulonephritis or diabetic kidney disease, even if the patients are normotensive. If this effect can be extrapolated to patients on dialysis therapy, it would suggest that these agents can slow the decrease in kidney function even in those with normal blood pressure. In both studies of ACE inhibitors and ARBs, the salutary effect of the drugs on RKF was independent of changes in blood pressure.99,100 One study specifically targeted patients with a blood pressure of at least 120/70 mm Hg.99 Although average entry blood pressure was high, it is not clear whether normotensive patients were involved in these studies and whether the agents had an effect in this subset of patients (Table 8).
Insults (Table 7) to RKF in patients with CKD also should be considered insults to RKF in PD patients and should be avoided when possible. Other drugs, events, and interventions that worsen kidney function in patients with CKD also should be expected to worsen RKF in patients on dialysis therapy. Potential insults are listed in Table 7; this list should not be considered all inclusive. Whereas it is reasonable to make the assumption that exposure to these potential nephrotoxins might harm RKF in PD patients, there is little high-grade evidence to prove it.
Retrospective analyses of RKF found that previous episodes of PD peritonitis are associated with faster kidney decline.89,101 This could be the result of the inflammation of the peritoneum itself, drugs used to treat the infection, or associated ECF volume depletion. A general linear multivariate model also implicated the use of aminoglycosides, separate from the rate of peritonitis, as an associated factor.89 A retrospective study of RKF found that patients for whom peritonitis was treated with aminoglycosides had a greater decrease in kidney function compared with those treated with other less-nephrotoxic antibiotics.102 However, the most recent retrospective analysis could not detect a difference in the slope of decrease in GFR in PD patients with peritonitis treated with or without gentamicin.103 The data therefore are not strong and are somewhat contradictory. However, if an antibiotic without the nephrotoxic potential of an aminoglycoside can be used in its place without compromising antibacterial efficacy, it is still recommended to do so.
Other agents that should be avoided are nonsteroidal anti-inflammatory drugs (NSAIDs), including cyclooxygenase-2 (COX-2) inhibitors. These drugs may be particularly harmful under conditions of preexisting kidney insufficiency or diminished kidney blood flow. This setting, of course, applies to RKF in patients on dialysis therapy; thus, this may represent a group particularly vulnerable to the nephrotoxic effects of COX-2 inhibitors. Conventional analgesia, such as acetaminophen, should be used in dialysis patients with noninflammatory pain. Other drugs to consider are low-dose opiates (watching for constipation) and short courses of oral or intra-articular corticosteroids for acute inflammatory noninfectious arthritis.
Intravenous or intra-arterial dye can be nephrotoxic, especially in patients with antecedent kidney dysfunction, particularly diabetic nephropathy. Again, there is no reason to expect that this risk is less for RKF in patients on dialysis therapy. In dialysis patients with kidney function, the decision to administer a dye load should not be taken lightly. The indication for the dye study should be reviewed, and alternative studies that do not use dye should be sought. The patient who must undergo the study should be well hydrated at the time, and the smallest volume of the least nephrotoxic dye should be used. Whether pretreatment with N-acetylcysteine is helpful in decreasing the incidence and severity of dye nephrotoxicity is controversial in patients with CKD; there are even fewer data for patients on dialysis therapy.104,105 Furthermore, there are no studies examining volume expansion as a method of protecting RKF in patients on dialysis therapy who must undergo contrast studies. However, given the low cost and favorable side-effect profile of N-acetylcysteine, consideration should be given to pretreating patients with this agent before the dye study, and it also would seem reasonable to ensure that volume depletion is not present.
As in any patient with unexplained deterioration in kidney function, both prekidney and postkidney causes should be ruled out. Given that the mean age of patients starting dialysis therapy is increasing, prostatic hypertrophy with urinary obstruction must be considered in men with sudden deterioration in function. Episodes of ECF volume depletion are associated with a decrease in urine volume and function106,107 and should be avoided unless necessary to keep the patient out of CHF.
PD is associated with low bone turnover. In PD patients, there is a good chance of hypercalcemia as a result of aggressive therapy with oral calcium or calcitriol and vitamin D analogs. The resulting increase in serum calcium concentration could be nephrotoxic; thus, hypercalcemia should be avoided.
Finally, many patients who start on (or return to) PD therapy after a “failed” kidney transplant have significant residual function in the transplanted kidney. It is unclear whether patients should continue to receive immunosuppressive therapy, particularly with agents other than calcineurin inhibitors, in an attempt to prolong this RKF. A recent decision analysis suggested that the benefit of continued immunosuppression outweighed the risk when CCr was greater than approximately 1.5 mL/min.108 However, this conclusion remains to be validated by clinical studies.
Whether urine volume, small-solute clearance, or some other kidney-related factor is responsible for the decrease in mortality associated with RKF, it is important to have some measure of this residual function. It is impracticable to use exacting tests to calculate this, such as inulin clearance or radionucleotide measurements. The average of urea nitrogen and CCr has been shown to have a reasonable approximation of RKF.109 However, the accuracy of this measurement depends on the careful collection of 24-hour urine. Especially in patients with very little function, inaccuracy in the timing of the collection can lead to incorrect results. Accuracy perhaps can be improved by the collection of a 72-hour sample and dividing the result by 3110; however, this is a time-consuming and cumbersome process. Patients will need to be instructed on the careful collection of 24-hour urine and make it a habit to bring these collections as part of the regular clinic visit.
Use of ACE inhibitors and ARBs may add to the cost of medications for patients. In addition, there is a risk for cough, particularly with ACE inhibitors. There also is a theoretical risk for hyperkalemia, although this has not been found in studies to date.