In a PD prescription, there are certain general considerations.
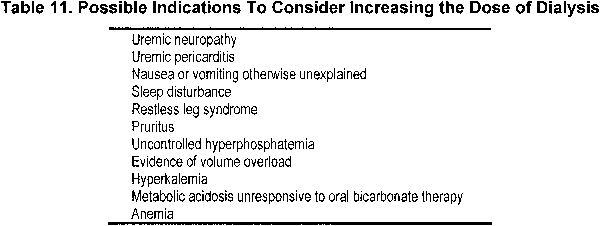
2.1 Regardless of delivered dose, if a patient is not thriving and has no other identifiable cause other than possible kidney failure, consideration should be given to increasing dialysis dose (see Table 11).
2.2 In a patient with minimal RKF, a continuous (rather than intermittent) 24 h/d of PD dwell PD prescription should be used to maximize middle-molecule clearance.
2.3 If either peritoneal Kt/Vurea is at least 1.7 or 24-hour urine output is less than 100 mL, monitoring of RKF is not required for monitoring the dose of PD. However, periodic measurement of RKF may be of value in this group of patients for the reasons noted in Table 12.
2.4 All measurements of peritoneal solute clearance should be obtained when the patient is clinically stable and at least 1 month after resolution of an episode of peritonitis.
2.5 More frequent measurements of either peritoneal urea clearance or RKF should be obtained when clinically indicated (see Table 13).
2.6 When calculating Kt/Vurea, one should estimate V from either the Watson or Hume equation in adults. In the absence of evidence, use of the patients' ideal or standard (rather than actual) weight should be considered in the calculation of V.
2.7 The determination of peritoneal CCr is of little added value for predicting risk for death; therefore, for simplicity, adequacy targets are based on urea kinetics only. Peritoneal creatinine excretion rate may be used to monitor estimates of muscle mass over time.
2.8 During the monthly evaluation of the PD patient, nutritional status should be estimated. Serum albumin levels should be monitored, and when obtaining 24-hour total solute clearances, estimations of dietary protein intake (DPI; such as nPNA) should be measured.
Regardless of delivered dose, if a patient is not thriving and has no other identifiable cause other than possible kidney failure, consideration should be given to increasing the dialysis dose. There are many reasons that a dialysis patient fails to do well. Often, failure to do well on PD therapy relates more to comorbidity174 or complications (such as peritonitis) than to adequacy. However, close examination of the 2 randomized trials of CAPD patients comparing small-molecule clearances gives some support to increasing dialysis in the symptomatic patient. In the ADEMEX Study, lower prescription (total average Kt/Vurea of 1.8 versus 2.27) resulted in more deaths from CHF (13.4% versus 5.7% in the intervention group; P < 0.05) and uremia, hyperkalemia, and/or acidosis (12.2% versus 5.1% in the intervention group; P < 0.05). More patients in this unblinded study were withdrawn because of uremia in the control group (5% versus none in the intervention group). Another randomized trial confirmed these results. Six percent of those in the group with total Kt/Vurea of 1.5 to 1.7 were withdrawn because of “inadequate” dialysis versus none in the groups with total Kt/Vurea of 1.7 to 2.0 and greater than 2.0 (P = 0.002 comparing all 3 groups). In addition, another 8% were withdrawn because of inadequate ultrafiltration in the group with Kt/Vurea of 1.5 to 1.7 versus 4% and 1% in the groups with total Kt/Vurea of 1.7 to 2.0 and greater than 2.0, respectively (P = 0.012). The group with the lowest clearance also required more erythropoietin. In the opinion of the Work Group, if a patient has symptoms possibly attributable to inadequate dialysis, such as anorexia, nausea, anemia, and hyperkalemia, or if volume overload is present, consideration should be given to increasing the dialysis dose.
Two additional indications listed in Table 11 for increasing the dialysis dose are uremic pericarditis and neuropathy. Tradition suggests that if a pericardial rub develops in a dialysis patient, the intensity of dialysis should be increased. This is an area that is poorly studied. Uremic neuropathy also is not very well understood, but if it develops, the dose of dialysis should be increased, with attention to removal of middle molecules.
There are no convincing data to support increasing small-molecule clearance to improve nutritional status or QOL. A study randomly assigned CAPD patients on 3 CAPD exchanges to continue on this prescription (n = 42) or to increase to 4 exchanges (n = 40). Peritoneal Kt/Vurea stayed constant at 1.56 in the 6-L group and increased from 1.59 to 1.92 in the 8-L group. Net ultrafiltration was better in the latter group, as was nPNA, which increased from 1.10 to 1.24 (P = 0.05), but there was no change in serum albumin level.175 Another study increased the prescribed PD in 23 patients with a subsequent increase in peritoneal Kt/Vurea from 1.62 to 1.96, which was associated with an increase in serum albumin level from 3.55 to 3.83 g/dL.176 It was unclear whether the increase in serum albumin level was caused by improved volume status (weight actually decreased and nPCR did not change). Others found that increasing the PD prescription to offset the loss of RKF did not result in an improvement in protein intake.177 The NECOSAD reported that RKF correlated significantly with many parameters of the Kidney Disease-QOL (physical functioning, role limitations, social functioning, mental health, vitality, bodily pain, general health, symptoms, effect of kidney disease on daily life, sleep disorders, and overall health rating). Peritoneal clearances correlated with none of these.44 In the ADEMEX Study, QOL was similar in the groups randomized to the higher small-molecule clearance relative to the lower target (total average Kt/Vurea of 1.8 versus 2.27).40 In addition, patients with a serum albumin level less than 3 g/dL at the start had similar survival whether administered the higher or lower delivered dose. In confirmation of these results, patients randomized to a total Kt/Vurea of 1.5 to 1.7 had outcomes in respect to composite nutritional index and serum albumin level similar to patients with higher total Kt/Vurea .39 In an observational study of anuric Chinese CAPD patients, serum albumin level did not correlate with Kt/Vurea .67 Mean Kt/Vurea of these patients was 1.72 to 1.73 during the course of the 2-year observation. Serum albumin level in PD patients appears to be linked to inflammation and volume overload.49,57,178,179
In summary, if the patient appears to have uremic signs and symptoms, the PD prescription can be changed to increase small-molecule clearance. However, there are no convincing data that this will lead to better nutritional status, survival, or QOL.
In a patient with minimal RKF, continuous (rather than intermittent) (24 h/d of PD dwell) PD prescription should be used to maximize middle molecule clearance. Middle-molecule clearance, in contrast to small-molecule clearance, is much more a function of total time of dialysis rather than dialysate flow rate. Because evidence suggests that middle molecules, such as β-amyloid, contribute to joint and bone disease in the long term, it seems reasonable to maximize middle-molecule clearance in PD patients.β2-Microglobulin levels increased over time in the CANUSA Study and were associated with increased risk for death and hospitalization.65 Maximizing middle-molecule clearance is achieved best by performing continuous PD without dry periods. It is unclear whether starting PD with a “dry abdomen” as a form of incremental dialysis in patients with significant RKF has benefits (such as protection of the peritoneal membrane against continuous glucose exposure or potential enhancement of peritoneal immune function). Further research is needed in this area.
If either peritoneal Kt/Vurea is at least 1.7 or 24-hour urine output is less than 100 mL, monitoring RKF is not required for monitoring dose of PD. However, periodic measurement of RKF may be of value in this group of patients for the reasons noted in Table 12. A study of anuric patients on APD therapy with a 24-hour prescription71 found that predictors of survival were age; SGA score of C, indicating malnutrition (RR, 6.97; P = 0.006); and diabetes, but not time-averaged total CCr. Anuria was carefully defined in this study as 24-hour urine volume less than 100 mL and GFR (determined by using the average of urea and creatinine kidney clearance based on 24-hour collection of urine) less than 1 mL/min/1.73 m2. Patients with any RKF continued to provide 24-hour urine collections; they had an average kidney clearance of 1.92 L/wk/1.73 m2 at the start of the study and 0.59 L/wk/1.73 m2 at 24 months. In this study, peritoneal clearances were repeated every 2 months until the planned targets were reached, and then every 6 months.
Similarly, in the ADEMEX Study, patients brought in repeated clearances (urine and peritoneal) every 2 months until the target was achieved; then the frequency was reduced to every 4 months.38 In the Hong Kong randomized trial, after initial adjustment of the prescription to get the patient into the target total Kt/Vurea range (which, in some cases, required reducing the peritoneal dose), the clearance (kidney and peritoneal) was repeated in 4 to 6 weeks, then every 6 months until the study ended (15 months after the last patient recruitment).39 Average kidney Kt/Vurea decreased steadily over time and was less than 0.1 by the study end point in all 3 groups. The CAPD prescription was continuously adjusted upward to enable patients to stay within the total Kt/Vurea targets of 1.5 to 1.7, 1.5 to 2.0, and greater than 2.0.
In an observational study, urine volume was determined every 2 months at clinic visits by having the patient measure daily urine volume for the 7 days before the visit; this was normalized to 1.73 m2.48 Actual measurement of kidney and peritoneal clearance was performed every 6 to 12 months. Total fluid removal (ultrafiltration plus urine volume) was a strong predictor of survival (RR, 0.90 for every 100 mL/24 h; P < 0.01). RKF also was a strong predictor of survival (RR, 0.41 for every increase in RKF of 1 mL/min/1.73 m2; P < 0.01).
Because a total Kt/Vurea greater than 1.7 has not been associated with clinical benefits, if this goal is achieved through PD, there would seem to be no need for measuring RKF. However, given the importance of RKF for survival, measuring 24-hour urine volume may focus attention on remaining kidney function. Furthermore, in the opinion of the Work Group, for patients who have persistent edema, measuring sodium losses in urine and effluent may help in management. However, there are few data for this.
There is considerable heterogeneity in the decrease in RKF in PD patients.89 Therefore, measurement of RKF seems warranted to monitor this important predictor of outcome.89,101 Peritonitis may have a negative impact on RKF; therefore, reassessing RKF after an episode of peritonitis would appear reasonable.
All measurements of peritoneal solute clearance should be obtained when the patient is clinically stable and at least 1 month after resolution of an episode of peritonitis. Peritonitis transiently changes the patient to a high transporter and decreases ultrafiltration per dextrose concentration used. Therefore, a dialysate clearance obtained close to an episode of peritonitis may either overestimate (because of the high transport status) or underestimate (because of the decrease in convection from decreased ultrafiltration) clearance of small molecules. Therefore, it appears best to defer a collection until 1 month or more after peritonitis. A change in prescription may require time for the patient to reach equilibrium; therefore, a delay in performing the collection is warranted.
More frequent measurements of either peritoneal urea clearance or RKF should be obtained when clinically indicated (see Table 13). For a patient with failure to thrive with no alternative explanation, repeated clearance of urine and peritoneal effluent may determine whether uremia is contributing to the problem. With the development of intravascular volume depletion, inadvertent use of NSAIDs, or other intercurrent events, a PD patient may lose significant RKF such that the PD prescription is no longer adequate. A decrease in dialysate dextrose concentration may result in decreased ultrafiltration and decreased clearance, leading to uremia. Overzealous blood pressure control also may lead to loss of RKF. Last, the patient may change the timing of the exchanges (ie, shortening some and lengthening others excessively), leading to inadequate dialysis. Repeating the clearance, if clinically indicated, may uncover these potential problems. Consideration should always be given to nonadherence with the prescription if the patient is not doing well. Nonadherence may be investigated by assessing the supplies ordered, as well as home supply inventory and analysis of the cycler memory system (if available).
When calculating Kt/Vurea , one should estimate V from either the Watson or Hume equation in adults. In the absence of evidence, use of the patient's ideal or standard (rather than actual) weight should be considered in the calculation of V. For the patient close to or at dry weight, the Watson or Hume equation is acceptable.180,181 The Watson equations tend to underestimate total body weight.182,183 In underweight patients, it also seems sensible to adjust the clearance for ideal body weight. An international cross-sectional study184 examined the nutritional status of 224 CAPD patients, of whom 71 were anuric, defined as no urine output. When parameters of severely malnourished patients were adjusted to desired weight, nPCR was decreased (0.76 versus 0.98 for well-nourished patients), as was Kt/Vurea (1.40 versus 1.68). These results suggest it is important to normalize V to calculate Kt/Vurea in malnourished patients. In amputees, total body water (TBW) must be calculated by determining the percentage of body weight lost in the amputation (using a nomogram) and dividing actual weight by percentage of body composition remaining, applying this weight with the nonamputated height to the Watson formula to determine the proportion of body water. This proportion then is multiplied by actual weight to obtain V.185
However, the correct determination of V for overweight patients is unclear.186 The Watson formula overestimates TBW in obese patients and underestimates it in overhydrated patients.183 Body size does not affect dialysate to plasma (D/P) ratio of small solutes.187
Determination of peritoneal CCr is of little added value for predicting risk for death; therefore, for simplicity, adequacy targets are based on urea kinetics only. Peritoneal CCr may be used to monitor estimates of muscle mass over time. Total CCr in patients with RKF is much a reflection of RKF. In the absence of RKF, CCr seems to add little to the use of urea clearance. A study examined 912 PD patients by using the USRDS data set, as well as by questionnaires completed by centers, and found that kidney urea clearance (but not dialysate urea clearance) was predictive of 12-month mortality. Neither kidney nor dialysate CCr were predictive.43 However, peritoneal and kidney creatinine excretion is a good measure of muscle mass and may be used to measure this sequentially if it seems appropriate.188,189
During the monthly evaluation of the PD patient, nutritional status should be estimated. Serum albumin levels should be monitored and when obtaining 24-hour total solute clearances, estimations of DPI (such as nPNA) should be measured. Nausea, vomiting, and appetite suppression are acknowledged symptoms of uremia. Uremic patients tend to have decreased DPI,190 and spontaneous DPI decreases as renal rGFR decreases to less than 50 to 25 mL/min.191 These tendencies may be exacerbated during the period before the initiation of dialysis therapy when many patients are not only anorexic, but also are acidotic and often treated with low-protein “renal protective” diets. As a result, patients may show signs of protein malnutrition when they present for dialysis. Dialysis itself is associated with unique metabolic and nutritional problems. PD patients may have a decreased appetite and early satiety192,193 and typically lose 5 to 15 g of protein and 2 to 4 g of amino acids per day in their dialysate.194 These losses amount to a net loss equivalent to 0.2 g protein/kg/d and tend to be higher in rapid transporters than low transporters. These losses are increased transiently during episodes of peritonitis,195 at times doubling after even a mild episode. Studies of patients with CKD stage 5196-199 showed that some of the most important predictors of patient risk for death are such surrogates of nutritional status as serum albumin level, SGA score, and DPI estimate. Hence, it would be appropriate to monitor and maintain normal nutritional status in patients on PD therapy. However, it is important to note that there have been no prospective randomized trials that evaluated a patient's RR for death when 2 different levels of a surrogate for nutritional status were compared as the target intervention.
A patient's RR for death correlates with surrogates of nutritional status. It is well recognized that such markers of “nutritional status” as serum albumin level, estimation of DPI, and SGA score200 are influenced by many additional clinical parameters other than nutrition-related ones; therefore, they must be treated as imperfect surrogates for nutritional status of a patient. For example, in an individual PD patient, the significance of an isolated serum albumin level must be viewed with caution. An isolated level does not necessarily predict nutritional status. Levels must be followed up over time and interpreted in the context of other patient-related issues, such as peritoneal membrane transport type, total solute clearance, volume status, presence of chronic liver disease, presence of comorbid diseases, and any inflammatory state.
Most (95%) nitrogen intake in humans is in the form of protein. Therefore, when the patient is in a steady state (not catabolic or anabolic), total nitrogen excretion multiplied by 6.25 (there are ~6.25 g protein per gram of nitrogen) is thought to be an estimation of DPI.201 Estimated DPI is calculated from urea nitrogen appearance in dialysate and urine. Multiple equations have been derived, some of which have been validated in CAPD (but not nightly intermittent PD [NIPD]) patients (protein equivalent of total nitrogen appearance [PNA] = protein catabolic rate [PCR] + protein losses). These estimations initially were called the PCR. However, PCR actually represents the amount of protein catabolism exceeding synthesis required to generate an amount of nitrogen that is excreted, ie, PCR is a net catabolic equivalent. Thus, because these calculations are based on nitrogen appearance, the term is more appropriately called the protein equivalent of nitrogen appearance, or PNA. Following up a patient's nPNA (discussed next) over time is a way to estimate DPI over time to ensure adequate nutritional status. A baseline PNA should be obtained during training. These should be recalculated every 4 to 6 months by using the same 24-hour dialysate and urine collections used to monitor solute clearances. One cause of a decreasing nPNA would be decreasing DPI at times because of suboptimal total solute clearance.
For comparison purposes, it is recommended that PNA be normalized for patient size (ie, nPNA). What patient weight (actual, standard, ideal) to use for that normalization is controversial. Depending on the weight used in calculating nPNA, there may or may not be a statistical relationship between clinical evidence of malnutrition and nPNA values less than target. PNA normalized by actual weight tends to be high or may appear to be increasing over time in malnourished individuals if normalized (divided) by a progressively smaller malnourished weight compared with the patient's baseline weight.202 It therefore is the opinion of the Work Group that ideal weight be used for the normalization process.
Data from the Centers for Medicare and Medicaid Services (CMS) Clinical Performance Measures (CPM) Project for the year 2000 found that, in long-term PD patients, mean nPNA was 0.95 ± 0.31 g/kg/d, normalized creatinine appearance rate was 17 ± 6.5 mg/kg/d, and mean percentage of lean body mass was 64% ± 17% of actual body weight.203
There is some controversy about what amount of DPI, in terms of grams of protein per kilogram of body weight, is needed to maintain a positive nitrogen balance in PD patients. Early studies suggested that DPI of at least 1.2 g/kg/d was needed to maintain nitrogen balance,204,205 a value considerably higher than that recommended for healthy individuals. The NKF KDOQI guidelines recently recommended DPI for long-term PD patients of 1.2 to 1.3 g/kg/d.35 Two cross-sectional studies suggested that patients who show no signs of malnutrition seem to eat less protein (0.99206 and 0.88207 g/kg/d, respectively). Results likely are caused by variations in the patient populations studied, historical dietary patterns, and amounts of RKF present. Therefore, several investigators proposed that daily protein intake in these patients should be in the range of 0.9 to 1.1 g/kg/d.208,209 If values are less than this amount, one should consider looking for potential causes of decreased DPI, such as intentional low DPI, gastroparesis, comorbidity, chronic inflammation, and suboptimal small-solute removal.
There is little evidence from prospective RCTs that increasing small-solute removal results in an improvement in surrogate markers for nutritional status. In both the ADEMEX Study and Hong Kong trials, the intervention groups (higher small-solute clearance) did not have an improvement in surrogate measurements of nutritional status (albumin level and PNA). If surrogate markers for nutritional status suggest that the patient's nutritional state is declining, one should consider evaluation for new comorbidity, additional dietary evaluation, dietary supplements, and, if no other cause is identified, an increase in dialysis dose.
There is a marked lack of high-quality studies of PD patients examining different doses of PD. Only 2 randomized trials have been performed, both in CAPD patients. There are no randomized trials of different doses of small-molecule clearances in APD patients. There also are no studies comparing initiation of PD therapy with a cycler at night and a dry day versus CCPD. There are no randomized trials targeting different levels of blood pressure control. There are only 2 randomized trials of interventions to protect RKF and none examining the effect of different prescriptions (especially APD versus CAPD) on RKF. Control of middle molecules is believed to be important to prevent long-term complications, but studies of this are mostly lacking.
Obtaining a clearance in PD patients is very dependent on the cooperation of the patient. The patient must bring the used dialysate to the dialysis unit. This may be difficult for elderly or weak patients unable to lift heavy objects or those with limited transportation. If the patient is told to sample the effluent and record the weight (for CAPD) or drain volume (for APD), the center is dependent on the patient providing the correct numbers. Furthermore, on the day of the clearance, the patient is more likely to do the proper full prescription. Therefore, the measurement, at best, is that of that particular day's dialysis and not necessarily reflective of average clearance. To some extent, use of a cycler with a mechanism of monitoring the use of the cycler and time on the cycler could be used.210 This cycler is not universally available and increases the cost of treatment.