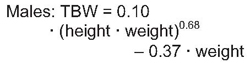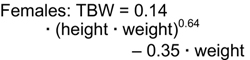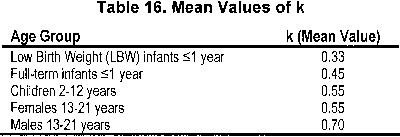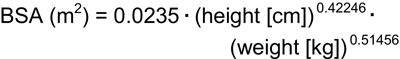NKF KDOQI GUIDELINES
Clinical Practice Guidelines and Clinical Practice Recommendations
2006 Updates
Hemodialysis Adequacy
Peritoneal Dialysis Adequacy
Vascular Access
I. CLINICAL PRACTICE GUIDELINES FOR PERITONEAL DIALYSIS ADEQUACY
CLINICAL PRACTICE RECOMMENDATIONS FOR GUIDELINE 6: PEDIATRIC PERITONEAL DIALYSIS
6.1 Dialysis initiation:
- 6.1.1 Dialysis initiation should be considered for the pediatric patient when GFR is 9 to 14 mL/min/1.73 m2 BSA and should be recommended when GFR is 8 mL/min/1.73 m2 or less. GFR can be estimated by either averaging the measured creatinine and urea clearances by using a timed urine collection, using the Schwartz formula, or using a timed urine collection to determine CCr after a dose of cimetidine. Dialysis therapy initiation should be considered at the greater estimated GFR levels when the patient's clinical course is complicated by the presence of malnutrition, fluid overload, hypertension, hyperkalemia, hyperphosphatemia, acidosis, growth failure/decreasing height velocity, or neurological consequences of uremia. Before dialysis is undertaken, these conditions should be shown to be persistent and refractory to medication and/or dietary management.
6.2 Modality selection:
- 6.2.1 The decision regarding the selection of PD as a dialysis modality for the pediatric patient should take a variety of factors into account, including patient/family choice, patient size, medical comorbidities, and family support.
6.3 Solute clearance targets and measurements:
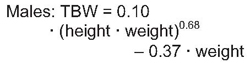
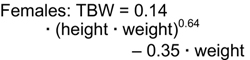
6.4 Preservation of RKF:
- 6.4.1 Techniques that may contribute to the preservation of RKF in pediatric patients receiving PD should be incorporated as a component of dialysis care whenever possible.
- 6.4.1.1 Nephrotoxic insults in those with normal or impaired kidney function should be assumed, in the absence of direct evidence, to also be nephrotoxic in patients on PD therapy who have RKF and therefore should be avoided.
- 6.4.1.2 Aminoglycoside antibiotics should be avoided whenever possible to minimize the risk for nephrotoxicity, as well as ototoxicity and vestibular toxicity.
- 6.4.1.3 “Prekidney” and “postkidney” causes of a decrease in RKF should be considered in the appropriate clinical setting.
- 6.4.1.4 Infections of the urinary tract should be treated promptly.
- 6.4.1.5 Diuretics should be used to maximize urinary salt and water excretion.
- 6.4.1.6 An ACE inhibitor or ARB should be considered in a PD patient who requires antihypertensive medication and has RKF.
6.5 Writing the PD prescription:
- 6.5.1 In addition to solute clearance, QOL, ultrafiltration/volume control, and possibly the clearance of middle molecules should be considered when writing the PD prescription.
- 6.5.1.1The patient's dialysis schedule and QOL as it relates to such issues as school and work attendance/performance should be taken into account when designing the dialysis prescription.
- 6.5.1.2 To optimize small-solute clearance, minimize cost, and possibly decrease the frequency of exchanges, one should first increase the instilled volume per exchange (target range, 1,000 to 1,200 mL/m2 BSA; maximum, 1,400 mL/m2 BSA), as tolerated by the patient, before increasing the number of exchanges per day. The volume of the supine exchange(s) should be increased first because this position has the lowest intra-abdominal pressure. Objective evidence of patient tolerance may require assessment of IPP.
- 6.5.1.3 The patient's record of PD effluent volume should be reviewed monthly, with particular attention to the drain volume from the overnight dwell of CAPD and daytime dwell of CCPD.
- 6.5.1.4 Factors to be considered when attempting to optimize total body volume include:
a. Dietary sodium and fluid restriction may be implemented in patients unable to maintain euvolemia/normotension with dialysis alone.
b. In patients with RKF, diuretics may be preferred over increasing the dialysate dextrose concentration to achieve euvolemia.
c. Drain volume should be optimized after the overnight dwell of CAPD and the daytime dwell(s) of CCPD to maximize solute clearance and ultrafiltration volume.
d. In patients who are hypertensive or in whom there is evidence of volume overload, ultrafiltration generally should be positive for all daytime or nighttime exchanges.
e. An effort should be made to determine the lowest possible dialysate dextrose concentration required to achieve the desired ultrafiltration volume.
- 6.5.1.5 To optimize middle-molecule clearance in patients who have minimal RKF, the PD prescription should preferentially include the use of CCPD with dwells 24 h/d or CAPD. This is recommended even if small-molecule clearance is above target without the longer dwell.
- 6.5.1.6 The use of NIPD (eg, no daytime dwell) can be considered in pediatric patients who are clinically well, whose combined dialysis prescription and RKF achieves or exceeds the target solute clearance, and who are without evidence of hyperphosphatemia, hyperkalemia, hypervolemia, or acidosis.
6.6 Other aspects of the care of the pediatric PD patient:
- 6.6.1 All children on PD therapy with anemia should follow the KDOQI Guidelines for Management of Anemia that pertain to pediatrics.231
- 6.6.2 Management of dyslipidemias for prepubertal children on PD therapy should follow recommendations by the National Cholesterol Expert Panel in Children and Adolescents.232 Postpubertal children or adolescents on PD therapy should follow the pediatric recommendations provided in the KDOQI Clinical Practice Guidelines for Managing Dyslipidemia in CKD.233
- 6.6.3 All children on PD therapy should follow the pediatric-specific recommendations provided in the KDOQI Clinical Practice Guidelines for CVD in Dialysis Patients and the KDOQI Clinical Practice Guidelines on Hypertension and Antihypertensive Agents in CKD.158,166
- 6.6.4 All children on PD therapy should follow the recommendations provided in the KDOQI Clinical Practice Guidelines for Nutrition in Chronic Renal Failure.35
RATIONALE
Dialysis Initiation
The gold standard for measurement of GFR is inulin clearance, but this technique is impracticalto perform clinically. Whereas the use of such radioisotopic measures as chromium-51, iothalamate sodium 125 I, and technetium 99-DTPA are alternative measures to inulin, these techniques are expensive, require multiple blood samples, and are not ideal for frequent monitoring.234,235
A measured CCr requires a timed urine collection, most often 12 to 24 hours in duration. The accuracy of the assessment as a means of estimating GFR is complicated by the need for a complete urine collection and that creatinine secretion results in overestimation of GFR, especially at lower levels of kidney function.236,237 At lower levels of GFR, accuracy is improved by measuring both creatinine and urea clearances on the same timed urine collection and averaging the values to obtain the estimated GFR.238,239
The accuracy of the GFR estimate by CCr can be increased by the provision of cimetidine to the patient before the timed urine collection.239 A study of children showed that as a result of cimetidine's capacity to block the kidney's tubular secretion of creatinine, its use in a formal outpatient protocol is associated with GFR results that approximate those obtained with inulin.240,241
The Schwartz formula also overestimates GFR, especially at lower GFR levels, and provides a less accurate means of estimating the target clearance for dialysis consideration than what can be determined with a complete timed urine collection.242 However, recent pediatric data show that a GFR of 15 mL/min/1.73 m2 or less estimated by using the Schwartz formula had an excellent negative predictive value for a measured GFR of 20 mL/min/1.73 m2 by using iothalamate clearance.243
Because a timed urine collection often is not possible for smaller non–toilet-trained children, reliance on a serum creatinine–based formula, such as the Schwartz formula, is essential in this subset of patients. The Schwartz formula contains a cofactor that accounts for patient sex and age to incorporate estimates of lean body mass. The Schwartz formula is calculated in the following manner4, 242:
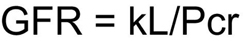
where GFR is expressed in milliliters per minute per 1.73 m2, L represents body length in centimeters, Pcr is plasma creatinine in milligrams per deciliter, and k, a constant of proportionality, is a function of urinary creatinine excretion per unit of body size (see Table 16).
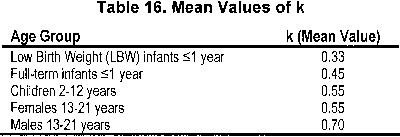
Finally, a variety of signs and symptoms may be present in the pediatric patient with CKD stage 4 (GFR, 15 to 29 mL/min/1.73 m2) that are not routinely associated with the presence of uremia, but that remain unresponsive to medical and/or dietary therapy. A trial of dialysis may on occasion result in marked clinical improvement.
Modality Selection
PD is the preferred initial long-term dialysis modality worldwide for the pediatric patient with CKD stage 5.244, 245 Its use is particularly advantageous in the very small patient for whom maintenance of a functional and complication-free vascular access can be problematic. The provision of PD, often in association with the use of an automated cycling device, also facilitates regular school attendance for most age-appropriate children.245 The use of PD is preferred over HD when there are contraindications to the use of anticoagulation, in children who have cardiovascular instability, and in children who live far from a pediatric HD center.
However, there are absolute and relative contraindications to the use of PD in children that include the following246:
Absolute contraindications:
- Omphalocele
- Gastroschisis
- Bladder extrophy
- Diaphragmatic hernia
- Obliterated peritoneal cavity
- Peritoneal membrane failure
Relative contraindications:
- Inadequate living situation for home dialysis
- Lack of appropriate caregiver
- Impending/recent major abdominal surgery
- Imminent living-related donor transplantation (within 6 months of dialysis initiation)
Recognition of the burden of care for families that coexists with the provision of this home therapy is paramount so that appropriate support systems may be put in place.247 Assessment of the patient's and caregiver's perception of QOL may aid in this process .246A
PD Solute Clearance Targets and Measurements
The clinical status of the pediatric patient should be monitored closely as an important qualitative means of determining whether the patient is receiving an adequate quantity of dialysis. Irrespective of the delivered dose of dialysis, adequate dialysis likely is provided if the patient's clinical status is characterized by adequate growth, blood pressure control, and nutritional status; avoidance of hypovolemia and sodium depletion; and adequate psychomotor development.246,248
Clinical manifestations of inadequate dialysis may include the following:
- CHF
- Hyperphosphatemia/excessive serum calcium × phosphorus product
- Uncontrolled hypertension/hypervolemia
- Overt uremia (uremic pericarditis, pleuritis)
- Repeated hyperkalemic episodes
- Clinical or biochemical signs of malnutrition or wasting
- Poor school performance
Factors contributing to inadequate dialysis include:
- Loss of RKF
- Prescription inadequate for peritoneal membrane transport characteristics
- Reduced peritoneal surface area caused by extensive intra-abdominal adhesions
- Loss of membrane solute transport/ultrafiltration capacity because of peritonitis
- Noncompliance with PD prescription
- Poorly functioning PD catheter
Current clinical opinion supports the recommendation that the target “delivered” solute clearance in pediatric patients should meet or exceed adult standards. The term “delivered” refers to the actual dose the patient is receiving based on measurement, in contrast to an estimated value using a kinetic modeling program.151,156 Data from pediatric and adult patients found serum albumin level to be a predictor of patient survival, and a Kt/Vurea of 1.8 or greater in adult PD patients has been associated with better serum albumin values.55,249 The ADEMEX Study did not show a clinical benefit associated with Kt/Vurea greater than 1.7/wk in adult CAPD patients, whereas other studies provided evidence for a recommended minimal Kt/Vurea greater than 1.7/wk and an optimal Kt/Vurea of 1.8/wk based on survival data in anuric adult CAPD patients.38,39,70 No similar large-scale studies have been performed in children. Pediatric studies have presented data suggestive of a correlation between patient outcome (especially growth) and total solute clearance; however, the number of patients in these and other pediatric studies is small and the potential role of RKF can be confounding, and thus data correlating solute clearance to outcome cannot be considered definitive.250,251 Nevertheless, it is recommended that solute clearance assessments take place at least every 6 months in all cases and that more frequent assessments be conducted when dialysis clearance may have been compromised (eg, after peritonitis), there is a progressive loss of RKF, or there is clinical evidence of inadequate dialysis.
Historically, both Kt/Vurea and CCr have served as measures of dialysis clearance. In addition, the averaged urea and CCr from a timed urine collection has been recommended as the most accurate means to estimate RKF and remains a preferred approach to estimate GFR when considering dialysis therapy initiation.238,239 Nevertheless, determination of dialysis and urine Kt/Vurea alone currently is recommended for follow-up based upon the simplicity of the calculation and because studies of adult patients on PD therapy have not provided evidence of a benefit in terms of patient outcome when expressing clearance in any manner other than Kt/Vurea.252,253 The age-related differences in the residual urine volume of children with CKD stage 5 precludes duplication of the adult preference to universally characterize the presence of RKF as urine volume greater than 100 mL/d.
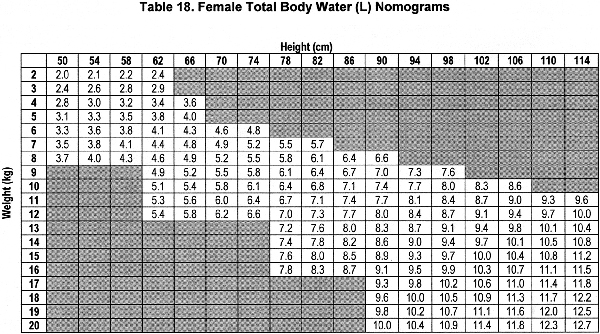
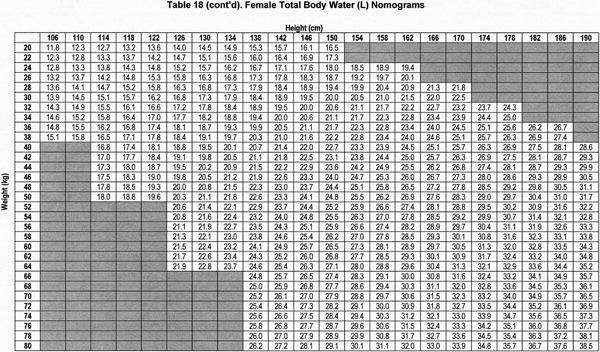
Accurate estimation of TBW or V is a critical component of the dialysis prescription in PD. Because gold-standard isotope dilution techniques are laborious, cost-ineffective, and not widely available, anthropometric prediction equations based on height and weight commonly are used to determine TBW.254 During childhood, complex changes in body composition occur that necessitate the use of appropriate allometric formulae. Whereas such equations have been established in healthy populations, recent studies showed that the use of these equations routinely overestimates TBW in pediatric patients receiving PD.255-257 Conversely, the recent determination of TBW by heavy water (H2O18 or D2O) dilution in 64 pediatric patients receiving PD has allowed for the development of TBW prediction equations that perform equally well in male and female, North American and European, obese and nonobese, and growth-retarded and normally sized children.148
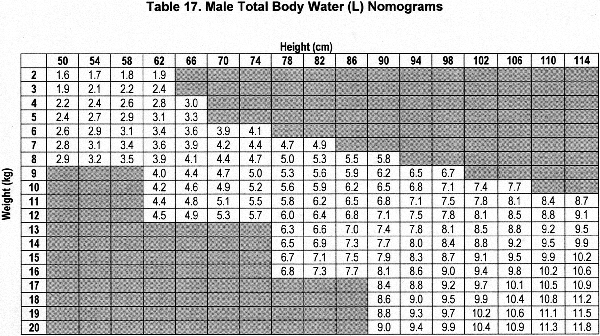
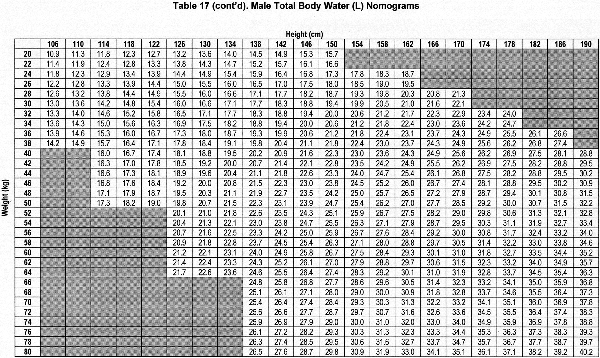
The sex-specific nomograms designed to estimate TBW, which are based upon the prediction equations, are shown in Table 17 and Table 18.
Because the height • weight parameter also predicts BSA, use of the Gehan and George equation for BSA allows for TBW-estimating equations that can be simplified, but with slightly less precision, compared with the best fitting equations to:
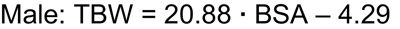
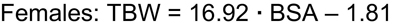
Whereas several approaches to the calculation of BSA are used in pediatrics, the Gehan and George equation for BSA was derived from the greatest number of study subjects.258,259 The Gehan and George equation is as follows:
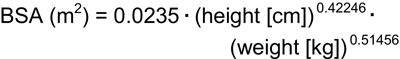
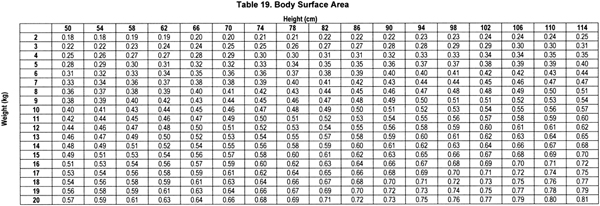
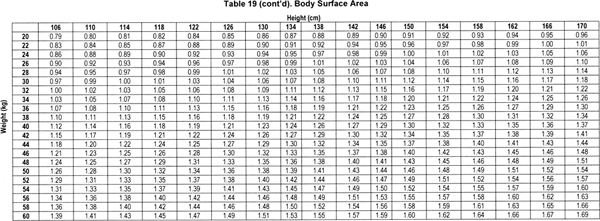
Based on this equation, BSA can be determined by height and weight by referring to Table 19.
Preservation of RKF
There are no large-scale studies in pediatrics that provide evidence of a correlation between RKF and patient outcome in children receiving PD. However, in a single-center observation of a pediatric PD population, it was shown that superior growth velocity occurred in a group of children with RKF versus a group of children without RKF despite the achievement of similar mean total solute clearance in the 2 groups of patients.250 Thus, it is possible that growth, as well as achievement of solute clearance goals, benefits from RKF and emphasizes the need to prevent nephrotoxic insults whenever possible. In addition, there is evidence that pediatric patients on PD therapy lose RKF at a slower rate than patients on HD therapy.260
While there is no experience regarding the use of ACE inhibitors or ARBs in children with CKD stage 5 similar to that in adults, use of an ACE inhibitor in children with CKD has been associated with marked slowing of kidney deterioration.99,100,261 In the setting of CKD stage 5, close monitoring for the presence of hyperkalemia is mandatory when an ACE inhibitor or ARB is used.262
Writing the PD prescription
Both CAPD and APD are used by children, and the prescription designed for either modality is best tailored to the needs of the individual patient. APD is the preferred PD modality in children, in large part because its use is characterized by freedom from procedures during the daytime hours.245,263 The pediatric PD patient's QOL and the influence that the dialysis prescription has on it is an issue that should be reassessed regularly because of the impact that CKD can have on the child's overall development. Although there are not yet any validated measures of QOL designed for the pediatric CKD stage 5 population, the PedsQL™ 4.0 Generic Core Scales and the Child Health Questionnaire have both been used successfully in the pediatric dialysis population.246A,264,265
Pediatric data have provided evidence that the prescription of an exchange volume that results in an exceedingly high IPP may result in patient intolerance and poor ultrafiltration.266 Whereas the target range for the exchange volume of patients older than 2 years is 1,000 to 1,200 mL/m2 BSA, the initial prescribed volume should be somewhat lower for smaller infants (~600 to 800 mL/m2 BSA). A stepwise increase in volume as tolerated by the patient usually is possible.
While the limitation of dietary sodium in children may have a positive influence on total body volume, this recommendation should be instituted with caution in patients with high RKF and/or dialysis-related sodium losses. Salt depletion may result in hypotension and impaired growth.267
The removal of “middle molecules” and low-molecular-weight proteins ideally also should be taken into account in the prescription process because of the influence it may have on clinical outcome, especially in patients without RKF.248 However, few data exist on the topic in pediatrics, prompting it to currently have a minor role in prescription considerations for children.268
Although the PD prescription is characterized most often by 24-hour dwells, in some circumstances, NIPD without the use of a daytime dwell can be used effectively. Its use requires that the patient's clinical status be monitored closely and consideration be given to a 24-hour dwell prescription if NIPD is not fully effective. This recommendation has been made previously by the European Pediatric PD Working Group.269
LIMITATIONS
No large-scale prospective study has been conducted in children on PD therapy that correlates solute removal (PD and RKF) with patient outcome. This precludes the ability to make an evidence-based recommendation regarding the target solute clearance.
Few data are available for children that compare the impact of RKF versus peritoneal solute removal on patient outcome.
Although data are available from the adult CKD stage 5 population showing the benefit of ACE-inhibitor and ARB therapy as a means of preserving RKF, no similar pediatric data are available.
The ability to assess the QOL of the pediatric PD patient and his or her family is limited by the absence of a QOL tool that has been validated in the pediatric CKD stage 5 population.