Several pharmacological agents and nonpharmacological manipulations of the HD prescription have been examined for potential efficacy as adjuvants to ESA treatment. Studies are not available to address the use of pharmacological or nonpharmacological adjuvants to ESA treatment in patients with ND-CKD and PD-CKD.
3.3.1 L-Carnitine: In the opinion of the Work Group, there is insufficient evidence to recommend the use of L-carnitine in the management of anemia in patients with CKD.
3.3.2 Vitamin C: In the opinion of the Work Group, there is insufficient evidence to recommend the use of vitamin C (ascorbate) in the management of anemia in patients with CKD.
For the purposes of these recommendations, we consider adjuvants to ESA therapy to be therapeutic agents or approaches that aim to enhance responsiveness to ESA therapy in iron-replete patients. The target patient population may include both ESA-hyporesponsive and relatively responsive patients, although the focus of interest often is the hyporesponsive patient. A positive response to an adjuvant treatment consists of either an increase in Hb level at a given ESA dose or the attainment and maintenance of a specific Hb level at a lower ESA dose (see Executive Summary).
L-Carnitine
In the opinion of the Work Group, there is insufficient evidence of efficacy to recommend use of L-carnitine in the management of anemia in patients with CKD. The role of carnitine deficiency in the pathogenesis of the anemia of CKD, if any, is unclear. Levocarnitine (L-carnitine) is a carrier molecule involved in the transport of long-chain fatty acids into the mitochondria, where they are oxidized to produce energy. L-carnitine is also thought to be involved in the metabolic conversion of acyl coenzyme A, which accumulates in patients with renal failure and is toxic to cells, to the less toxic acyl carnitine.191 Deficiency of carnitine in patients on maintenance HD therapy was demonstrated nearly 30 years ago.192 L-carnitine, which has been studied primarily when administered IV to HD patients, has been postulated to have beneficial effects on ESA-hyporesponsive anemia, HD-related hypotension, myocardial dysfunction, impaired exercise tolerance and performance status, muscle symptoms, and impaired nutritional status. However, no pathogenic mechanism by which carnitine deficiency might contribute to anemia or provoke ESA hyporesponsiveness has been conclusively elucidated. Furthermore, the therapeutic mechanism by which L-carnitine administration might improve anemia or enhance ESA responsiveness has not been determined. Finally, no consistent relationship between baseline plasma carnitine levels, anemia, and response to L-carnitine administration has been demonstrated.193,194
In considering L-carnitine administration, the Work Group confined evaluation of efficacy outcomes to RCTs in which the effect of IV L-carnitine on Hb level and ESA dosing had been reported in patients with CKD. Thus, Work Group conclusions and the resulting guideline statement 3.3.1 address the limited use of L-carnitine as an ESA adjuvant in patients with CKD. Guideline 3.3.1 does not address use of L-carnitine for potential nonhematologic indications.
In the United States, Medicare coverage for L-carnitine is available for patients who have been on dialysis therapy for at least 3 months, have a plasma free carnitine level less than 40 µmol/L, and have “erythropoietin-resistant anemia…that has not responded to standard erythropoietin dosage…and for which other causes have been investigated and adequately treated.”195 Hyporesponsiveness to ESAs is not specifically defined other than having a persistent Hct less than 30% despite erythropoietin dosage that “is considered clinically appropriate to treat the particular patient.”
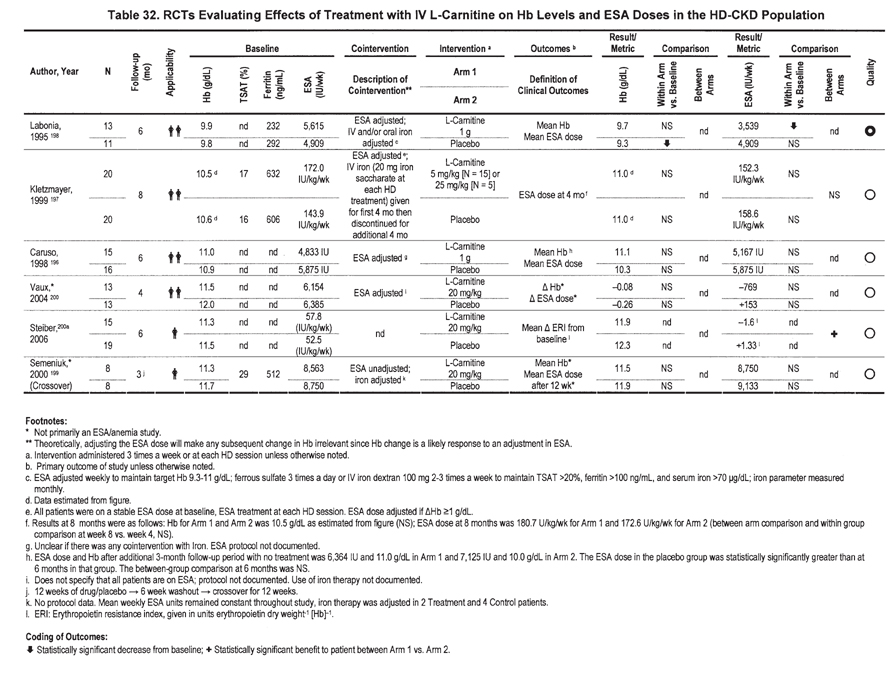
The statement There is insufficient evidence to recommend use of L-carnitine in the management of anemia in patients with CKD is supported by results from 6 available RCTs of IV L-carnitine administration to ESA-treated HD patients (Table 32). 196-200 No RCTs are available in patients with PD-CKD or ND-CKD (Table 32). Anemia was the primary study outcome in only 3 of these studies.196-198 No available RCTs were judged to be of high quality. Five of the 6 RCTs were judged to be of low quality. The RCTs were characterized by small numbers of enrolled patients, short duration of observation, concomitant use of IV and oral iron, adjustments in ESA dosage, high dropout rates, and uncertainty about specific ESA and/or iron dosing during the study. Between-group comparison, the result least subject to bias, was available in only 2 studies; there was no difference in either Hb level or ESA dose outcomes in 1 of these studies, and in the other, the comparison was only of erythropoietin resistance index (ERI). Serious limitations in method quality, important inconsistencies, major uncertainty about the directness and applicability of results, imprecise data, and a probability of bias rendered the overall quality of evidence very low (Table 33). None of the studies specifically enrolled patients with anemia and ESA hyporesponsiveness or identified a specific subset of patients particularly likely to respond to L-carnitine administration. Therefore, whether L-carnitine enhances the effect of ESA therapy in such patients is not known. The Work Group found no specific evidence of adverse drug effects associated with IV L-carnitine in patients with CKD. The absence of high-quality evidence for efficacy and safety supports the opinion of the Work Group that there is insufficient evidence to recommend use of L-carnitine in the management of anemia in patients with CKD.
The conclusion of the Work Group differs from those of selected previous reports. A meta-analysis concluded that L-carnitine administration was associated with higher Hb levels and lower ESA doses in ESA-treated patients with HD-CKD.201 However, the meta-analysis included only 3 of the 6 RCTs examined by the Work Group and incorporated results from studies outlined in 3 abstracts, but that were never published in peer-reviewed journals. An NKF Carnitine Consensus Conference recommended the use of IV L-carnitine in selected ESA-hyporesponsive dialysis patients.202 However, the consensus conference lacked systematic data abstraction and analysis of method quality.
Previous guideline development processes using systematic evidence review and rigorous evaluation for method quality have reached conclusions consistent with the current guideline statement, there is insufficient evidence to recommend the use of L-carnitine in the management of anemia in patients with CKD. These include NKF-KDOQI Guidelines for Nutrition in CKD,203 previous NKF-KDOQI anemia guidelines,2 and the Revised EBPGs for Anemia in CKD.16
Finally, although oral L-carnitine has also been studied as an ESA adjuvant, no RCTs are available. Direct comparison of IV to oral L-carnitine has not been reported in HD or other CKD patients.
Vitamin C (Ascorbate)
In the opinion of the Work Group, there is insufficient evidence to recommend use of vitamin C (ascorbate) in the management of anemia in patients with CKD. Vitamin C has been reported to increase the release of iron from ferritin and the reticuloendothelial system and increase iron utilization during heme synthesis.204,205 Although many HD patients may have plasma ascorbic acid levels less than the normal range,206,207 whether this reflects a clinically significant deficiency is uncertain; in other studies, ascorbic acid levels have been normal or elevated.208 It has been suggested that 150 to 200 mg of vitamin C daily is needed to normalize vitamin C levels in most HD patients.206
Several anecdotal reports, small case series,194,209 and nonrandomized studies (primarily in HD patients with iron overload, elevated serum ferritin levels, and functional iron deficiency) using IV vitamin C in doses of 100 to 500 mg 3 times weekly have suggested a possible beneficial effect.210-213 Plasma levels of ascorbic acid were not measured in most of the studies in which vitamin C was administered as an adjuvant to ESA therapy.
Four RCTs of vitamin C in ESA-treated HD patients have been reported (Table 34). Although the uncontrolled studies mentioned tended to focus on a possible role of IV vitamin C in patients with HD-CKD with iron overload and functional iron deficiency, only 1 RCT included patients with functional iron deficiency.214 One RCT included patients with iatrogenic iron overload.215 None of the studies specifically included patients with ESA hyporesponsiveness. These studies have not shown consistent benefit of IV vitamin C in either within-group or between-group comparisons.
Oral vitamin C, which can augment absorption of iron from the GI tract, has been evaluated as an adjunct to ESA therapy in small uncontrolled studies.216-218 Oral and IV vitamin C were compared in 1 recent RCT in a small number of HD patients for 8 weeks; in a larger number of patients, oral vitamin C or no treatment were compared for 3 months.219 There was no significant difference within or between groups in Hb levels or ESA doses in either comparison.
The long-term safety of IV ascorbic acid in HD patients remains undefined, with secondary oxalosis being the primary concern,220,221 although this was not described with short-term use in any of the studies described. Plasma oxalate levels increase with IV vitamin C administration. A risk for calcium oxalate supersaturation in plasma recently was reported in HD patients administered IV vitamin C.222 Aside from the potential for systemic oxalosis, concern is also warranted because a pro-oxidant effect of high-dose vitamin C, either directly or through its effects on mobilization of iron, has been reported.223,224
In summary, the Work Group concluded that serious method limitations and important inconsistencies render the quality of available information on vitamin C efficacy very low (Table 35). At the same time, information on the potential serious adverse effects (oxalosis) of chronic vitamin C administration is altogether lacking. Thus, the Work Group concluded that there is insufficient evidence to recommend use of vitamin C (ascorbate) in the management of anemia in patients with CKD. None of the RCTs enrolled patients with ESA hyporesponsiveness, and only 1 enrolled patients with functional iron deficiency214; therefore, whether IV vitamin C enhances the effect of ESA therapy or iron metabolism in such patients is not known.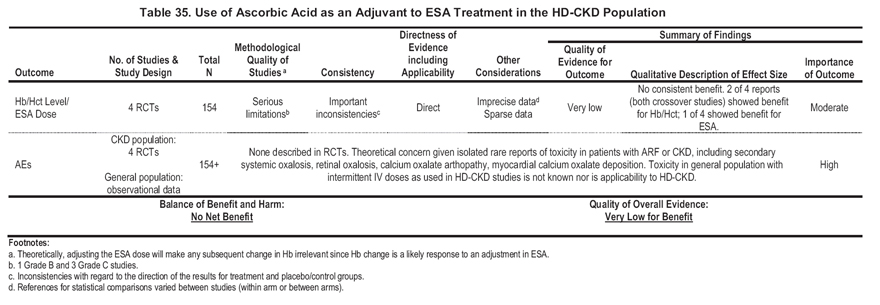
In addition to the lack of definitive evidence of efficacy and safety, there is no evidence that clinical outcomes—such as reduced hospitalizations, improved cardiovascular status, and reduced mortality—are improved in patients for whom IV vitamin C treatment is initiated as an ESA adjuvant. Whereas the putative mechanism of action of IV vitamin C as an ESA adjuvant is an increase in the release of iron from ferritin and the reticuloendothelial system and increased iron utilization during heme synthesis, none of the studies reviewed showed reduced utilization of administered iron therapy. IV vitamin C has not been evaluated in patients with PD-CKD.
Therefore, given the low quality of evidence for efficacy and unresolved concerns for serious AEs of chronic administration, the Work Group concluded that there is insufficient evidence to recommend use of vitamin C (ascorbate) in the management of anemia in patients with CKD.
Androgens
Androgens should not be used as an adjuvant to ESA treatment in anemic patients with CKD. Before the availability of epoetin therapy, androgens were used regularly in the treatment of anemia in dialysis patients despite the need for intramuscular (IM) injection and a variety of AEs, including acne, virilization, priapism, liver dysfunction, injection-site pain, and risk for peliosis hepatis and hepatocellular carcinoma. Proposed mechanisms of action of these drugs include increased erythropoietin production from renal or nonrenal sites, increased sensitivity of erythroid progenitors to the effects of erythropoietin, and increased red blood cell survival.
Three RCTs explored a possible role for androgens in combination with ESA therapy in HD patients (Table 36). All were small short-term studies, currently recommended Hb levels were not achieved, and in 2 of the studies, the ESA doses used were lower than those used in most patients with HD-CKD on chronic ESA treatment. The studies did not enroll patients with ESA hyporesponsiveness, so it is not known what effect, if any, androgens would have in such patients. It is unclear whether any enhanced erythropoietic effect caused by androgens would confer clinical benefits that outweigh the potentially significant AEs of androgens or the effects of simply allowing patients to remain at somewhat lower Hb levels without androgens. Short-term and long-term toxicity of androgens limit their use, especially in women.
In short, evidence for efficacy of androgens is characterized by serious method limitations, important inconsistencies, and sparse data (Table 37). The Work Group, as a result, considered the quality of evidence to be very low. The Work Group judged mild to severe drug-related AEs in the target and general population as being highly important. Given the very low quality of evidence for efficacy and demonstrated AEs of androgen therapy, the Work Group concluded that androgens should not be used as an adjuvant to ESA treatment in anemic patients with CKD. (Strong Recommendation)
Other Pharmacological Agents Not Addressed in Guideline Statements
Statins
A growing body of literature indicates that there may be clinically important, non–lipid-lowering effects of the hydroxymethylglutaryl coenzyme A reductase inhibitors, or statins, including antiproliferative, anticoagulant, immunosuppressive, anti-inflammatory, antioxidant, and cytoprotective effects. Because a component of anemia in patients with CKD may be related to underlying inflammatory processes, a potential role for statins in enhancing epoetin therapy may be plausible. Only a single small retrospective study addressed statins as adjuvants to ESA therapy.225 Given the nature of this study, further investigation of the effects of statins as ESA adjuvants may be warranted, but their utility, if any, remains to be determined.
Pentoxifylline
One small uncontrolled open-label study of 16 patients with CKD (HD, PD, and transplant recipients) with ESA-resistant anemia evaluated the effect of pentoxifylline as an ESA adjuvant.226 Further studies may be warranted, but the utility, if any, of pentoxifylline as an adjuvant to ESA treatment in patients with HD-CKD remains to be determined.
Vitamin Supplements Other Than Vitamin C
Although deficiencies of vitamin B12 and folate are recognized causes of anemia and rarely are the basis for ESA hyporesponsiveness, there are no RCTs showing that supplementation with vitamin B12, folate, or other vitamins (in the absence of documented vitamin deficiency) is an effective adjuvant to ESA therapy. One RCT of ESA-treated HD patients did not find a benefit of pyridoxine (vitamin B6) as an ESA adjuvant.227
Summary
There was insufficient evidence for efficacy to recommend use of statins, pentoxifylline, and vitamin B12 and folate supplements (other than when used to correct documented vitamin deficiency) as adjuvants to ESA therapy in patients with CKD. Because these therapies are not in widespread use as ESA adjuvants, they are presented here primarily for general information, but were not considered by the Anemia Work Group for inclusion in Guidelines or CPRs addressing ESA adjuvants.
Modifications of Dialysis Treatments Not Addressed in Guideline Statements
The effects of modifications of the HD dialysis prescription and various components of the HD treatment on anemia in patients with HD-CKD have been studied. Unlike the pharmacological agents discussed, it is not likely that these dialysis treatment modifications would be undertaken for the primary purpose of enhancing ESA responsiveness, and they therefore were not considered by the Anemia Work Group for inclusion in Guidelines or CPRs addressing ESA adjuvants.
Distinguishing between the effects of increased HD dose (ie, urea reduction ratio [URR] or Kt/V) from effects of concomitant changes in membrane on response to ESA therapy is difficult because most studies compared different Kt/V levels using membranes of different types. The effects of dialysate composition, primarily in comparisons of standard bicarbonate dialysate to ultrapure dialysate, also have been evaluated. No RCTs have been reported that compared HD and PD (or different doses of PD) on anemia outcomes or ESA dose in ESA-treated patients.
HD Intensity (“dose”), Membrane Type, and Other Dialysis Modifications
A few studies have examined the relationship between dialysis dose and dialysis membrane type and anemia outcomes,228-236 with conflicting results. Because in most of these studies, patients in the higher versus lower Kt/V (or URR) groups were dialyzed with membranes of different composition and flux, the role of dialysis dose cannot be separated from the effects of membrane permeability or biocompatibility. One of these studies, which was not an RCT, compared different levels of Kt/V with the same membranes236 and observed an inverse correlation between ESA dose and Kt/V, but no relationship between Kt/V and Hb level, in HD patients treated with unsubstituted cellulose membranes. In a subsequent report, which also was not an RCT,235 it was suggested that this relationship holds only for patients with a Kt/V less than 1.33, and above this level, there was no correlation between Kt/V and ESA dose.
Three RCTs were performed in HD patients in which anemia-related outcomes were compared for high-flux and low-flux dialyzers.229-231 No difference in Hct or ESA responsiveness was found between groups. Assessment of these studies is complicated by the use of membranes of different composition, attainment of different Kt/V levels, short study duration, and inclusion of patients not on ESA therapy. In another RCT of HD patients who were unable to reach a target Hb level of 11 g/dL or greater with at least 200 U/kg/wk of recombinant human erythropoietin (rHuEPO), low-flux and high-flux polysulfone dialyzers were compared.228 Kt/V values were similar in the 2 groups at baseline and the end of the 6-month study. Hb levels were higher and ESA doses were lower in the high-flux group by 3 months and remained so to the end of the study.
Vitamin E
Vitamin E has been considered as a potential adjuvant to ESA therapy based on the consideration that antioxidant properties of vitamin E may prolong red blood cell life span in patients with CKD and anemia. Oral vitamin E has not been studied in prospective controlled trials of ESA-treated patients. Vitamin E–bonded dialyzers were studied in a single RCT in which the primary focus was the effects of a vitamin E–bonded hemodialyzer on carotid artery atherosclerosis and rheological properties of red blood cells.237 Mean ESA dose decreased after 1 year on the vitamin E–bonded dialyzers, but Hb levels and other important parameters, such as amount of iron administered, were not reported. The safety of vitamin E also must be considered: a recent meta-analysis in the non–kidney-disease population suggested that doses of 400 U/d or more of vitamin E were associated with an increase in all-cause mortality.238
Ultrapure Dialysate
Inflammatory cytokines are proposed to interfere with the erythropoietic effect of ESAs both directly and through impaired mobilization and utilization of iron. An inflammatory stimulus in HD patients may be endotoxin or bacterial contamination of dialysate. Standards for bacterial and endotoxin content of water used for dialysis and for dialysate vary around the world. For dialysate, recently revised voluntary standards (Association for the Advancement of Medical Instrumentation) include an upper limit for bacteria of 200 CFU/mL, and for endotoxin, of 2 EU/mL.239 In some countries, limits of 100 CFU/mL and 0.25 EU/mL for bacteria and endotoxin have been applied, respectively. Ultrapure dialysate has 0.1 CFU/mL or less of bacteria and less than 0.03 EU/mL of endotoxin.239, 240 Ultrapure dialysate is produced by generation of microbiologically purer water than used for standard dialysate, minimizing potentially contaminating biofilm, and use of ultrafilters. Some uncontrolled observations suggest that the response to ESA treatment may be enhanced by the use of ultrapure dialysate solutions.241 Three RCTs examined the effects of using online-produced or filtered ultrapure dialysate on anemia outcomes in HD patients.242-244 ESA doses were significantly decreased by up to 33%. The use of ultrapure dialysate typically was associated with lower C-reactive protein and interleukin 6 levels compared with standard dialysate, thought to be indicative of reduced inflammatory responses.
Hemodiafiltration
Hemodiafiltration (HDF) has been evaluated prospectively in a few RCTs with conflicting results. A small randomized study that compared acetate-free biofiltration and low-flux HD in ESA-treated patients suggested that ESA doses were lower with HDF.245 In a comparison of online HDF with high-flux HD, no differences in Hb levels or ESA dose were observed.246 Another study, in which only about 40% of patients were being treated with an ESA, compared HDF and high-flux HD. It found no difference in Hb levels or ESA doses.247 HDF with online production of pyrogen-free solutions also may have an advantage in terms or anemia outcomes compared with conventional solutions.248
Daily and Nocturnal HD
There are no RCTs comparing either daily HD or nocturnal HD with conventional intermittent HD for effects on anemia or ESA requirements. In the most recent report from a small nonrandomized comparison of short daily, long nocturnal, and conventional HD, only nocturnal HD patients had a statistically significant increase in Hb levels during 18 months; ESA doses also tended to increase, although the difference was not statistically significant.249 In 1 study, conventional HD patients with a baseline single-pool Kt/V of at least 1.3 were changed to short-daily dialysis 6 times per week with the same weekly dialysis time.250 Weekly Kt/V increased by 31%. In the patients studied for 12 months, mean ESA dose decreased 45% compared with baseline, with stable or increased Hb levels. Doses of ESA were trending upward between 6 and 12 months of observation. Other studies, none of which were RCTs, reported variable results.251-254 To our knowledge, it has not been studied whether changing from conventional HD to daily or nocturnal HD specifically enhances ESA responsiveness in ESA-hyporesponsive patients.
Peritoneal Dialysis
Although observational data have suggested that patients treated with PD may have lower ESA requirements than HD patients,255,256 no controlled trials have been reported comparing HD and PD or different doses of PD on anemia outcomes or ESA dose in ESA-treated patients.
Summary
Whereas the Anemia Work Group does not recommend changing patients with HD-CKD from standard bicarbonate dialysate to ultrapure dialysate for the purpose of enhancing ESA responsiveness, studies suggest that the use of ultrapure dialysate results in lower ESA doses in patients with HD-CKD. There is insufficient evidence at this time that other modifications in the HD prescription or various components of the HD treatment enhance ESA therapy.
We acknowledge the limitations of these guidelines. For lack of evidence, we do not address the potential adjuvant effect of pharmacological agents or alterations in dialysis prescription on anemia outcomes in patients without concomitant ESA therapy. Among patients receiving ESA therapy, available evidence is restricted to patients with HD-CKD. Among available ESA agents, evidence is restricted to use of epoetin alfa. As reported elsewhere in this document, a guideline needs to be based on both high- or moderate-quantity evidence and consistently demonstrated net medical benefit. Therefore, we considered appropriately designed, adequately powered RCTs to be the required foundation. Few such RCTs currently are available. Among available RCTs, only hematologic outcomes were assessed: no evidence exists to confirm the assumption that hematologic outcomes gained with adjuvant therapy share the same risk-benefit profile as those gained with ESA and iron therapy alone. Finally, although the primary motivation behind adjuvant therapy is to decrease cost by decreasing ESA doses, information on the comparative costs and benefits of ESA with and without proposed noniron adjuvants is lacking.