Anemia is relatively common after transplantation. Regular screening and careful evaluation of the multiple factors that can contribute to anemia after transplantation are recommended.
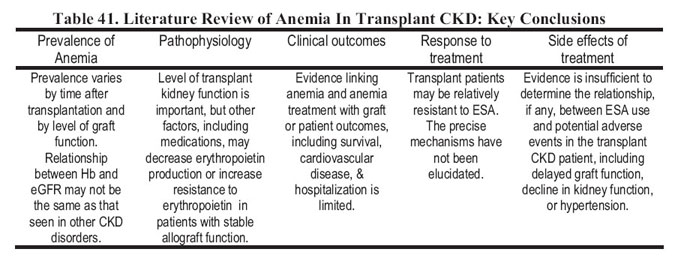
Table 41 summarizes the key features of posttransplantation anemia (PTA). As in other forms of CKD, the prevalence of anemia in transplant CKD clearly is associated with the level of allograft function. However, a number of other factors unique to transplant recipients may contribute to the development of PTA. There is little information regarding the association of anemia with either graft or patient outcomes. Similarly, there is limited information to suggest that the response to treatment with ESAs differs between patients with CKD with and without a transplant. Although there are reports of increased delayed graft function and hypertension with the use of ESAs, there is insufficient evidence to suggest that these agents should not be used in either the immediate posttransplantation or late posttransplantation period. Conversely, with the existing information, it is recommended that treatment guidelines for management of anemia in the general CKD population be followed in the transplant population.
Transplant recipients are a subgroup of patients with CKD with unique considerations with regard to anemia management.
Transplant recipients have variable exposure to CKD before transplantation. Patients with functioning transplants include preemptive transplant recipients, recipients with variable exposure to dialysis, and repeat and multiorgan transplant recipients. Consequently, the burden of comorbid disease varies among transplant recipients, and this may have important implications for the severity and management of anemia after transplantation.
Most transplant recipients have decreased kidney function. Among 40,963 transplant recipients studied in the United States between 1987 and 1996, mean eGFR achieved at 6 months after the time of transplantation was 49.6 ± 15.4 (SD) mL/min/1.73 m2.376 In a study of transplant recipients in the United States between 1987 and 1998, more than 70% of patients with allograft function at 1, 3, and 5 years after transplantation had CKD stage 3 or higher.377 Transplant function may change over time. Although the mean rate of GFR decline was only 1.66 ± 6.51 mL/min/1.73 m2 per year in a large cohort of long-term kidney transplant recipients, kidney allograft function may change rapidly; thus, frequent evaluation of kidney function and Hb level is desirable.376
Transplant recipients differ from most other patients with CKD because they are chronically exposed to immunosuppressive medications that may suppress erythropoiesis. The current KDOQI classification of CKD includes transplant recipients.4 A recent worldwide consensus statement of KDIGO also confirmed that the CKD classification system should be applied to transplant recipients and suggested to add the index letter “T” to the CKD stage category for these patients.377a Although many features distinguish transplant recipients from other patients with CKD, transplant recipients are clearly at risk for anemia because of decreased kidney function.
This section reviews the available information regarding the prevalence, pathophysiology, clinical correlates, and treatment considerations of anemia in kidney transplant recipients. The relevant considerations for anemia management before transplantation and after transplant failure also are discussed.
There is no accepted definition of anemia in transplant recipients, and variable definitions are used in the literature. A second important consideration is that the prevalence of anemia is dependent on the time of observation after transplantation.
During the early posttransplantation period, arbitrarily defined as the first 6 months after transplantation, anemia of varying degrees is very common. The prevalence and degree of anemia during this period are dependent on the pretransplantation Hb level, amount of perioperative blood loss, frequency of blood draws, iron depletion,378-383 persistence of uremia,382 endogenous erythropoietin levels,382,384-385 erythropoietin responsiveness,382, 384-387 and exposure to immunosuppressive agents.388,389
The time course of erythropoiesis after transplantation has been studied by a number of investigators,378-382,385,390,391 and reviews on this subject are available.389,391,392 A transient early peak of erythropoietin is detectable within the first 24 hours after transplantation, particularly in patients with delayed graft function, and is not associated with a measurable increase in Hb level. Within the first number of days after successful transplantation, a smaller, more sustained erythropoietin peak is detectable. This peak is associated with the subsequent onset of erythropoiesis and recovery of renal anemia during the next several months after transplantation.
The prevalence and degree of anemia in the immediate posttransplantation period also will be dependent on the pretransplantation Hb level. Despite the widespread availability of ESAs, anemia continues to be a significant issue in patients presenting for kidney transplantation. A study found that the prevalence of pretransplantation anemia (defined as Hct < 33%) was 41% in adults.393 In a study of pediatric transplant recipients, the prevalence of pretransplantation anemia (defined as Hct > 2 SDs less than age-specific means) was 67%.394 In the Transplant European Survey on Anemia Management (TRESAM), 4 cohorts of prevalent transplant recipients were defined by the duration of transplantation (6 months and 1, 3, and 5 years). Mean pretransplantation Hb levels in these cohorts were 11.9 ± 1.7 (SD), 11.7 ± 1.8, 11.2 ± 1.8, and 10.8 ± 1.8 g/dL, respectively. The higher mean Hb levels in cohorts that underwent transplantation more recently suggested an improvement in pretransplantation anemia management over time.395 In a study of adult first deceased-donor transplant recipients in the United States between 1995 and 2000, the prevalence of anemia before transplantation was described among the subset of patients who had Medicare as the primary payer for the 12 months before transplantation and who received ESAs before transplantation. Mean pretransplantation Hb levels recorded in the 6 months before transplantation by transplantation year were: 1995 (n = 2296), 10.5 ± 0.02 (SE) g/dL; 1996 (n = 2394), 10.7 ± 0.02 g/dL; 1997 (n = 2694), 10.9 ± 0.02 g/dL; 1998 (n = 2838), 11.0 ± 0.02 g/dL; 1999 (n = 2723), 11.4 ± 0.02 g/dL; and 2000 (n = 2857), 11.6 ± 0.02 g/dL. The higher Hb levels among patients who underwent transplantation more recently suggested an improvement in pretransplantation anemia management over time. Nonetheless, in 20% of patients, Hb levels decreased to less than the KDOQI target of 11 g/dL.395a
Table 42 summarizes the published studies describing the prevalence of PTA. Together, the existing literature indicates that PTA is highly prevalent. The results from the few longitudinal studies showed a very high prevalence of anemia in the early posttransplantation period; anemia appears to be least prevalent 1 year after transplantation and then increases in prevalence with time after transplantation. This increase possibly is related to declining allograft function. The available information suggests that the prevalence of anemia is greater in pediatric compared with adult patients.
A number of factors may cause PTA; some are shared with other patients with CKD, whereas others are unique to transplant recipients.398
Factors Shared With Other Patients With CKD
In general, the evaluation of PTA should parallel that among nontransplantation patients with CKD. The discussion regarding factors contributing to PTA that are shared with other patients with CKD is limited to the most common considerations and to unique considerations in the transplantation setting.
Kidney Function
Level of allograft function is clearly an important determinant of PTA. In the TRESAM, there was a strong association of anemia with kidney transplant function. Of 904 patients with an SCr level greater than 2 mg/dL, 60.1% were anemic compared with 29% of those with an SCr less than 2 mg/dL, P < 0.01. In a single-center study of 459 patients at least 6 months after transplantation, prevalences of anemia, defined as an Hb level less than 11 g/dL, among patients with CKD stages 1, 2, 3, 4, and 5 were 0%, 2.9%, 6.6%, 27%, and 33%, respectively.403
The association of level of allograft function with Hb level appears to vary with the time of observation after transplantation. One study found that, among patients with an eGFR greater than 90 mL/min/1.73 m2, 11% and 7% of patients had anemia at 6 and 12 months after transplantation, whereas among patients with an eGFR less than 30 mL/min/1.73 m2, at 6 and 12 months after transplantation, 60% and 76% were anemic, respectively.398 These findings suggest that factors in addition to level of allograft function may be important determinants of anemia, particularly during the early posttransplantation period.
Whether the association between anemia and level of kidney function differs in patients with CKD who did and did not undergo transplantation is uncertain. In a study of 23 renal transplant recipients with stable kidney function, 12 anemic patients were compared with the 11 nonanemic control patients.386 Of the 12 anemic patients, 10 had low erythropoietin levels suggestive of erythropoietin deficiency, 2 patients had higher than anticipated erythropoietin levels suggestive of erythropoietin resistance, whereas 5 of 11 nonanemic control patients had higher than expected erythropoietin levels. Thus, there appears to be significant variation in erythropoietin production and responsiveness in transplant recipients, which may alter the association between kidney function and anemia in transplant recipients. Other clinically evident examples of the dissociation between Hb level and kidney function in transplant recipients include posttransplantation erythrocytosis and, as in nontransplantation patients with CKD, the lower incidence of anemia among transplant recipients with polycystic kidney disease.395
Iron Deficiency
Iron deficiency may be an important factor in the development of anemia after transplantation.404 There is limited information regarding the prevalence of iron deficiency after transplantation. In a cross-sectional study of 438 prevalent transplant recipients, the prevalence of iron deficiency, defined as a percentage of hypochromic red blood cells of 2.5% or greater, was 20.1%. In another study of 439 prevalent transplant recipients, 41% of patients had a TSAT less than 20%, whereas 44% had a ferritin level less than 100 ng/mL.403
The prevalence of iron deficiency may be greater in the early transplantation period because of low pretransplantation iron stores in dialysis patients and increased iron utilization with the onset of erythropoiesis after successful transplantation.383 In 1 study, 24 of 51 patients were found to be iron deficient in the early posttransplantation period.381 In a prospective study of 112 transplant recipients, serum ferritin levels decreased from 109.6 µg/L (range, 21 to 4,420 µg/L) at transplantation to 54.9 µg/L (range, 2 to 1,516 µg/L) at 6 months after transplantation.405
Transplant-Specific Factors
Acute Rejection
Early acute rejection is reported to cause a sharp decrease in erythropoietin production and anemia.406 Insights into the molecular mechanisms involved in the development of anemia during allograft rejection have been elucidated from gene expression studies.407 Among 4 pediatric renal allograft recipients with acute rejection and anemia, a cluster of 11 genes involved in Hb transcription and synthesis, iron and folate binding, and transport were found to be downregulated. An additional mechanism for the development of anemia during rejection is thrombotic microangiopathy, which may develop during episodes of severe vascular rejection.
Medications
Immunosuppressive medications. The use of myelosuppressive medications for immunosuppression and antiviral prophylaxis or treatment may be important factors in the development of anemia after transplantation. Azathioprine and mycophenolate mofetil are myelosuppressive; therefore, anemia caused by these drugs often is associated with leukopenia and/or thrombocytopenia. Very rarely, PRCA may occur with the use of these drugs.388,408,409
Calcineurin inhibitors infrequently are associated with anemia. The most common mechanism for PTA associated with the use of calcineurin inhibitors is microangiopathy and hemolysis.410-414 The immunosuppressant OKT3 also has been associated with hemolytic uremic syndrome (HUS) and microangiopathy.415,416
Anemia was a significant AE in a phase III trial in which sirolimus was administered with cyclosporine and corticosteroids.417 A group reviewed the 10-year experience with sirolimus and reported a dose-dependent association of anemia with the drug in phase I and II trials.418 The association of sirolimus with anemia also recently was shown in single-center analyses.419 Sirolimus may inhibit erythropoiesis by interfering with intracellular signaling pathways normally activated after the binding of erythropoietin to its receptor, and sirolimus also may be associated with thrombotic microangiopathy.419,420
Antiviral and antimicrobial medications. A number of commonly used antivirals and antibiotics may cause anemia, including ganciclovir and trimethoprim-sulfamethoxazole.
Angiotensin-converting enzyme inhibitors and angiotensin II receptor antagonists. Angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs) may be associated with PTA. Anemic patients in the TRESAM had higher odds of receiving ACE inhibitors or ARBs (odds ratio, 1.55; 95% CI, 1.34 to 1.80; P < 0.001).395 In a single-center retrospective study, a significant curvilinear dose-response relationship was identified between ACE-inhibitor dose and Hct.400 The underlying mechanisms are complex and include inhibition of endogenous erythropoietin production, inhibition of angiotensin II–mediated stimulation of red blood cell precursors,421 and the generation of an erythropoiesis-inhibiting protein by ACE inhibitors.422
Infections and Malignancy
Anemia may be a feature of cytomegalovirus (CMV) infection. Parvovirus B19 infection has been reported in transplant recipients with anemia and may cause PRCA.423,424
Hemophagocytic syndrome (HPS) is a rare cause of PTA. The syndrome often is caused by infectious or neoplastic disease and is defined by bone marrow and organ infiltration by activated nonmalignant macrophages that phagocytose red blood cells. A retrospective analysis of 17 cases among deceased donor transplant recipients showed that the syndrome developed after a median duration of 52 days after transplantation. Fever was present in all patients and hepatosplenomegaly was present in 9 of 17 patients. Eleven patients had received antilymphocyte globulins in the 3 months before presentation. In 9 patients, HPS was related to viral infections (CMV, Epstein-Barr virus, and human heprus virus 6 and 8); other infections included tuberculosis, toxoplasmosis, and Pneumocystis carinii pneumoniae. Posttransplantation lymphoproliferative disease was present in 2 patients. The syndrome has a poor prognosis—8 of 17 patients died despite the use of anti-infectious therapy and tapering of immunosuppression.425
Hemolytic Uremic Syndrome
HUS may recur after transplantation and can result in allograft loss.426 De novo HUS may occur associated with the use of cyclosporine, tacrolimus, or OKT3. The syndrome also has been associated with CMV and influenza A infection in transplant recipients.427,428 The possibility that erythropoietic agents may be beneficial in patients with HUS after transplantation is suggested by observations from the nontransplantation population. Plasma from approximately 75% of patients with sporadic thrombotic thrombocytopenic purpura (TTP)/HUS induces apoptosis in cultured microvascular endothelial cells.429 The mechanism appears to be linked to induction of Fas (CD95) on cultured endothelial cells because the erythropoietin receptor is expressed on vascular endothelial cells.430,431 Erythropoietin prevents lipopolysaccharide-induced apoptosis in cultured endothelial cells, suggesting that erythropoietin may have a protective effect and may be of therapeutic benefit in TTP/HUS in the nontransplantation setting.432 The use of ESAs after transplantation in patients with HUS warrants further study.
Hemolytic Anemia Associated With Minor Blood Group A, Group B, Group O Incompatibility
Blood group A recipients receiving transplants from blood group O donors or blood group AB recipients receiving transplants from either group A or B donors may develop evidence of hemolysis caused by anti-A or anti-B antibodies of the donor or from autoantibodies produced by passenger lymphocytes.433-435
There is only limited information regarding the association of anemia with clinical outcomes in transplant recipients.
Transplant recipients are known to be at increased risk for cardiovascular events, particularly during the perioperative period. In a single-center study of 404 transplant recipients with diabetes between 1997 and 2000, patients with at least 1 monthly Hct level less than 30% during the first 6 months after transplantation had a significantly greater incidence of cardiovascular events compared with patients with monthly Hct values greater than 30%. In a multivariate analysis that also included patient age and history of ischemic heart disease (IHD), an increase in monthly Hct to greater than 30% was associated with a significantly lower risk for cardiovascular events (RR, 0.65; 95% CI, 0.33 to 0.91; P = 0.02).436
Among long-term transplant recipients, there is limited information regarding the association of anemia with adverse cardiovascular events. In a retrospective study of 638 transplant recipients between 1969 and 1999 who were alive and free of cardiac disease 1 year after transplantation, lower Hb levels were associated with an increased risk for de novo CHF (RR, 1.24/10-g/dL decrease in Hb; 95% CI, 1.10 to 1.39; P = 0.001).437 In a follow-up study, anemia was associated with increase in left ventricular mass (as measured by Cornell voltage on electrocardiogram) during the first 5 years after transplantation.438
In a recent study, 438 prevalent transplant recipients were followed up for a median of 7.8 years. Laboratory parameters obtained during a 4-week enrollment period in 1995 were tested for their association with all-cause mortality and allograft failure.439 The investigators did not identify an association between anemia (Hb < 10 g/dL) and all-cause mortality or graft survival. Compared with patients with hypochromic red blood cells less than 5%, patients with hypochromic red blood cells greater than 10% had an RR of 2.06 for all-cause mortality (95% CI, 1.12 to 3.79; P = 0.02).
In a retrospective single-center study of resource utilization among 220 kidney transplant recipients, patients with a higher Hct had a decreased risk for hospitalization (RR, 0.95/1% increase in Hct; 95% CI, 0.92 to 0.98; P < 0.001).440
Use of ESAs Before Transplantation
The issue of whether ESA use before transplantation is associated with delayed graft function after transplantation has largely been dispelled by the decreased incidence of delayed graft function in registry data over time despite the increased use of ESAs. A few small retrospective studies had suggested that ESA use may be associated with delayed graft function, presumably because of altered intrarenal blood flow during rewarming in patients with a higher Hct.441,442 Previous treatment with ESAs does not blunt the production of endogenous erythropoietin after transplantation or the ability to respond to endogenous erythropoietin.391,443
Early Posttransplantation Period
Erythropoietic agents are effective in correcting anemia during the early posttransplantation period. Two small prospective randomized studies to study the efficacy of ESAs during the immediate posttransplantation period have been performed.
One study randomized 14 transplant recipients to receive and 15 patients not to receive an ESA.387 The ESA (150 U/kg/wk) was started at an Hct less than 30% and was increased at weekly intervals by 30 U/kg/wk as long as Hct remained at less than 25%. Hct increased from a nadir of 22% ± 4% 2 weeks after transplantation to 30% ± 4% at week 4 and 36% ± 4% at week 6 (P < 0.001 and P < 0.0001 versus week 2, respectively). Corresponding values in the non-ESA group were 25% ± 6%, 28% ± 6% (P = NS), and 32% ± 6% (P < 0.05 versus week 2; overall ESA versus non-ESA, P = 0.038 by analysis of variance). The maximum ESA dose after transplantation was more than 2 times higher than that required before transplantation (197.1 ± 45.1 versus 85.0 ± 76.0 U/kg/wk; P < 0.05). The investigators concluded that ESAs could safely and effectively correct anemia during the first weeks after transplantation despite relative erythropoietin resistance.
In a recent study, patients were randomized to receive (n = 22) or not receive (n = 18) ESA, 100 U/kg 3 times per week, if Hb level was less than 12.5 g/dL.444 Time to reach an Hb level greater than 12.5 g/dL was 66.5 ± 14.5 versus 52.6 ± 23.7 days in the non-ESA and ESA groups, respectively (P = 0.05). After 3 months, Hb levels were not different between the non-ESA and ESA groups (12.6 ± 1.5 versus 12.0 ± 1.5 g/dL, respectively). In a Cox regression analysis, ESA use (RR, 7.2; P = 0.004) and dose (RR, 0.63; P = 0.04) were retained as independent variables predicting the time to reach an Hb level greater than 12.5 g/dL. In the ESA group, 14 of 22 patients reached the target Hb level of greater than 12.5 g/dL compared with 12 of 18 patients in the non-ESA group (P = NS). SCr levels were not different between groups. The investigators concluded that the use of ESAs in the immediate posttransplantation period had no relevant clinical impact on the correction of anemia after transplantation.
Together, these studies suggest that ESAs are effective in correcting anemia after renal transplantation. The dose of ESA required may be higher than that before transplantation. The studies were not designed to determine whether the correction of anemia was associated with improvement in clinical outcomes, resource utilization, or QOL. Further, few patients with delayed or impaired graft function were studied. Thus, whether there are clinically relevant benefits to the early correction of anemia after transplantation remains uncertain.
These studies did not report significant AEs associated with the use of ESAs, such as delayed graft function or hypertension. A recent study reported a significantly increased incidence of transplant renal artery stenosis among pediatric transplant recipients administered ESAs during the first week after transplantation. All patients were enrolled in a steroid-free immunosuppression protocol. Results of DNA microarray analysis showed a series of 12 genes that differentiated the patients who developed renal artery stenosis in this setting.444A
Erythropoietin modulates the cellular response to stress and may attenuate apoptosis and necrosis in various organs, including the kidney.445-451 Whether use of erythropoietic agents can minimize ischemic reperfusion injury, as shown experimentally, warrants investigation.
Late Posttransplantation Period
There are only a few uncontrolled studies describing the use of ESAs to treat late PTA.452-455 In the largest of these studies, in 40 patients with failing renal allografts, mean Hb levels increased from 78.9 ± 10.4 to 102.6 ± 18.4 g/L after 24 weeks of treatment with ESA, 50 U/kg 3 times per week.453 The increase in Hb level was associated with a significant improvement in QOL measures. Twelve patients returned to dialysis therapy. These patients all had poor allograft function, and the study could not exclude the possibility that treatment with ESA accelerated renal allograft decline. Although no change in systolic or diastolic blood pressure was noted, the need for antihypertensive medications was significantly increased in 18 patients. An increase in hypertension also was noted in another study.452 In a single-center study, correction of anemia was associated with a decreased rate of decline in allograft function.456
Together, these studies suggest that ESAs are efficacious and likely do not accelerate renal decline, but may aggravate hypertension. Whether patients in the late posttransplantation period require higher doses of ESAs to correct anemia compared with nontransplantation patients with CKD is unclear.457 A decreased response to ESAs among transplant recipients may be anticipated because of the use of myelosuppressive medications, chronic inflammation, or other factors. The propensity of specific immunosuppressive agents to affect the action of ESAs in kidney transplant recipients is unclear. There is an inverse correlation between ESA dose and CCr in transplant recipients, and a sudden change in Hct in ESA-treated patients may indicate a change in graft function.453 The response to ESAs is impaired during episodes of allograft rejection.407,458 In the TRESAM, the median dose among ESA-treated patients was 4,000 IU/wk (mean, 5,831 ± 4,217 IU/wk), but ESA-treated patients had lower Hb values than non–ESA-treated patients, suggesting that the ESA was underdosed and patients were ESA resistant.395
Posttransplantation Failure
Patients with allograft failure have a high mortality that is related primarily to CVD. The management of anemia among patients with failing allografts is suboptimal. In a study of patients initiating dialysis therapy after transplant failure in the United States between 1995 and 1998, mean Hct was 27.5% ± 5.9% and 67% had an Hct less than 30%. The use of ESAs at the time of dialysis therapy initiation was infrequent (35%).459 Anemia management may be more difficult in patients with transplant failure because of the presence of chronic inflammation and relative resistance to ESAs.460