Most kidney transplant recipients have CKD and hypertension. High blood pressure in kidney transplant recipients is a risk factor for faster progression of CKD and development of CVD.
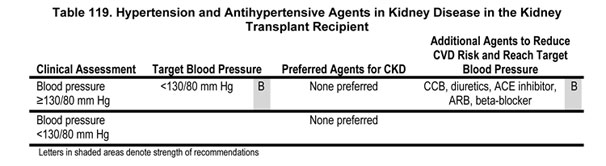
10.1 The target blood pressure in kidney transplant recipients should be <130/80 mm Hg (Guideline 7) (Table 119).
10.2 Patients with CKD in the kidney transplant should be treated with any of the following to reach the target blood pressure: CCB, diuretics, ACE inhibitor, ARB, or beta-blocker (Table 119).
Hypertension occurs in up to 80% of kidney transplant recipients. In the recently completed 5-year cadaveric kidney transplant trial comparing tacrolimus and cyclosporine, antihypertensive therapy was used in 81% of tacrolimus-treated and 90% of cyclosporine-treated recipients.472 Hypertension in the recipients appears to be a risk factor associated with reduced long-term graft survival. A large registry study showed that recipients with well-controlled blood pressure have improved long-term survival.473 Smaller, single-center studies also show a relationship with blood pressure control and long-term outcome.474-476 Although there appears to be a strong relationship between hypertension and long-term kidney transplant outcome, there are no clinical trials that assess the level of blood pressure control and long-term outcomes.
CKD in Kidney Transplant Recipients
Most kidney transplant recipients have CKD (either kidney damage or GFR <60 mL/min/1.73 m2 for >3 months). GFR is lower in individuals with a solitary kidney and is even lower in kidney transplant recipients because of toxicity from immunosuppressive agents used to prevent and treat rejection, such as cyclosporine and tacrolimus. Biopsy studies demonstrate pathological damage due to acute and chronic allograft nephropathy in virtually all transplant recipients, even if serum creatinine is normal. A recent study found abnormal glomerular permeability even in well functioning transplants.477 However, because albuminuria and abnormalities on urinalysis or imaging studies may not be sensitive to tubulointerstitial or vascular damage characteristic of allograft nephropathy, it is likely that many kidney transplant recipients will not have markers of kidney damage. Patients without markers of kidney damage, but with GFR <60 mL/min/1.73 m2 will be correctly classified as having CKD. However, patients without markers of kidney damage and GFR ≥60 mL/min/1.73 m2 may be incorrectly classified as not having CKD. The NKF-K/DOQI CKD Guidelines classify kidney transplant recipients without markers of kidney damage and with GFR >60 mL/min/1.73 m2 "at increased risk of CKD." Although Guideline 10 addresses hypertension and antihypertensive agents in kidney transplant recipients with CKD, the Work Group suggests that the recommendations also apply to hypertensive kidney transplant recipients at increased risk of CKD.
For this guideline, CKD was defined as Stages 1-4, according to the NKF-K/DOQI classification. The major causes of CKD in transplant recipients include (1) chronic allograft nephropathy (formerly termed chronic rejections); (2) toxicity due to cyclosporine or tacrolimus; (3) recurrent disease; and (4) transplant glomerulopathy (Table 120). In addition, all causes of CKD in native kidneys can also affect the transplant. Proteinuria is an important diagnostic finding in transplant recipients. Proteinuria is the hallmark of transplant glomerulopathy and recurrent disease due to glomerular diseases or diabetes, and can be associated with nephrotic syndrome. Lower levels of proteinuria are commonly seen in chronic allograft nephropathy. Because of the ease and safety of a kidney biopsy, there is generally a much lower threshold for performing invasive procedures to establish a diagnosis, especially if proteinuria is present.
Both immunological and nonimmunological factors appear to play a role in the progression of kidney disease in transplant recipients. As in diseases of the native kidneys, important factors appear to be hypertension and proteinuria. Hypertension and proteinuria are also risk factors for CVD, which is very common in kidney transplant recipients. Thus appropriate antihypertensive therapy may slow progression of kidney disease and reduce CVD risk in kidney transplant recipients as in patients with other causes of CKD.
Entries in summary tables comparing antihypertensive agents are grouped first by agent (ACE inhibitors first), then by control group medication (placebo then active control groups), then by methodological quality (highest first), then by applicability (widest first), then by study size (largest first).
Dihydropyridine calcium-channel blockers are associated with higher GFR after transplantation in short-term studies (Strong). Most of the major classes of antihypertensive medications have been studied and are effective in controlling hypertension in transplant recipients. Most transplant centers use dihydropyridine calcium-channel blockers for initial therapy, since these agents dilate the afferent arteriole, thereby ameliorating vasoconstriction of the afferent arteriole induced by calcineurin inhibitors (cyclosporine and tacrolimus).478 Randomized studies of the use of calcium-channel blockers for post-transplant hypertension demonstrate the effectiveness of this class of agents479-481 (Table 121). A recent randomized study comparing nifedipine and lisinopril demonstrated improved kidney outcomes (lower creatinine and improved GFR at 2 years) with the use of nifedipine.482 However, the study had limited follow-up, and it cannot be determined whether the improved GFR with calcium-channel blockers reflects the short-term hemodynamic effects of these agents or a long-term protective effect.
The available data on treatment of posttransplant hypertension are insufficient to recommend any class of antihypertensive agents as preferred agents for long-term therapy to slow the progression of kidney disease (Moderately Strong). Although it may be expected that ACE inhibitors and ARBs may be associated with beneficial effects on the progression of kidney disease, as in diabetic and nondiabetic kidney disease, there appears to be an increased risk of hyperkalemia and anemia in kidney transplant recipients receiving ACE inhibitors.489,490 In a recent retrospective study, discontinuation of ACE and ARB occurred in 25% of patients, but discontinuation for elevated creatinine, hyperkalemia, and anemia was only 9%.491 Because of the high incidence of ischemic heart disease (IHD), beta-blockers are often used as an adjunct agent for control of blood pressure. For the same reason, ACE inhibitors may also be used, despite the effects to lower GFR, raise serum potassium, and lower hemoglobin concentration. Diuretics may be useful since hypertension in cyclosporine treated patients may be sodium-dependent.492
Kidney transplant recipients have a high risk of CVD (Strong). Several studies document the high risk of CVD in kidney transplant recipients.493-495 There are several reasons for the high risk of CVD in kidney transplant recipients. Transplant recipients have a high prevalence of traditional CVD risk factors, such as hypertension and hyperlipidemia (see NKF-K/DOQI Clinical Practice Guidelines on Managing Dyslipidemias291b). In addition, since transplant patients have a history of kidney failure, they have also been exposed to CKD-related risk factors. Because of their high risk of CVD, kidney transplant patients are included in the highest-risk group for CVD. Two studies have shown the benefit of ACE inhibitors on LVH.480,496
Hypertension is a risk factor for CVD in kidney transplant recipients (Moderately Strong). Retrospective, observational studies show a higher level of blood pressure is associated with a higher risk for CVD.494,497 We found no long-term, randomized trials of blood pressure control in kidney transplant recipients. It was the opinion of the Work Group that target blood pressure should be <130/80 mm Hg, similar to recommendations in diabetic and nondiabetic kidney disease.
High blood pressure is a risk factor for faster progression of kidney disease in kidney transplant recipients (Moderately Strong). There have been no large, long-term randomized trials of the level of blood pressure control on kidney disease progression in kidney transplant recipients. A retrospective analysis of 29,751 kidney transplant recipients with blood pressure values at 1 year demonstrated an increased risk of graft loss with each 10 mm Hg increase in SBP over 140 mm Hg and DBP over 90 mm Hg (Fig 52).473 In addition, a single-center study of long-term kidney transplant survivors with a DBP of 89 to 99 mm Hg had a statistically increased rate of GFR decline compared to recipients with lower blood pressure.474 Another study has also demonstrated a graft survival advantage for transplant recipients with lower blood pressure readings at 1 year.475 In another study, the increased risk of graft failure for every 10 mm Hg increase in SBP was 1.15 (95% CI, 1.02 to 1.30) and for every 10 mm Hg increase in DBP was 1.30 (95% CI, 1.05 to 1.61).476
Fig 52. Collaborative Transplant Study. Relationship between systolic blood pressure and graft survival. Association of SBP at end of year 1 with subsequent graft survival in recipients of cadaveric kidney transplants. Ranges of SBP values in mm Hg and number of patient studies in the subgroups are indicated. The association of SBP with graft survival at 7 years was statistically significant (P < 0.0001).
Proteinuria after transplantation is a risk factor for graft loss and death (Moderately Strong). In a large, single-center, retrospective study, the risk of graft failure and death was greater in transplant recipients with proteinuria at 1 year.498 In this study, the relative risk of graft loss was 2.03; the relative risk of death was 1.98. Both cardiovascular and noncardiovascular causes of death were increased in recipients with proteinuria. Proteinuria after kidney transplantation was associated with a higher risk of graft loss (RR = 4.18), patient death (RR = 1.92), and CVD (RR = 2.45).499 Although we could find no long-term prospective data on treatment of proteinuria with ACE inhibitors or ARB, it seems reasonable to conclude that control of proteinuria and hypertension with these agents may have a favorable outcome on graft survival and mortality.
Guidelines for target blood pressure in transplant patients have been published.110,118 The American Society of Transplantation guidelines recommend a blood pressure target of <140/90 mm Hg. The British Renal Association guidelines recommend a target blood pressure of <130/80 mm Hg. Since most kidney transplant recipients have low levels of proteinuria, it was the opinion of the Work Group that the level of blood pressure control recommended for CVD prevention (<130/80 mm Hg) would be appropriate for slowing kidney disease progression. In patients with higher levels of proteinuria, a lower blood pressure goal and agents that lower proteinuria may be appropriate, as in nondiabetic kidney disease (Guideline 9).
Figure 53 and Table 122 summarize recommendations for kidney transplant recipients.
Fig 53. Hypertension and Antihypertensive agents in kidney transplant recipients. Superscripts refer to items in Table 122.
There are few randomized, controlled studies of the treatment of hypertension in kidney transplant recipients. Although it is clear from the data that dihydropyridine calcium-channel blockers are effective in controlling blood pressure and maintaining GFR in the posttransplant setting, long-term data are lacking on their effectiveness in slowing progression of kidney disease.
Achieving target blood pressure will require multiple antihypertensive medications, with frequent monitoring for efficacy and side-effects. ACE inhibitors and ARBs are effective in controlling blood pressure, but the long-term benefits on kidney disease progression and CVD risk are not well defined in this population.500 Concerns about anemia, hyperkalemia, and increased creatinine may limit their use.
Most kidney transplant recipients have CKD after transplantation. Kidney transplant recipients are an ideal population in which to study the effects of antihypertensive therapy on the progression of kidney disease and the risk of CVD. In these patients, the onset of kidney disease in the transplanted kidney is known, patients are treated in specialized centers, measurements of blood pressure and urine protein can be standardized by adherence to protocols, and study endpoints such as deterioration in GFR and graft loss are well defined.480,501-503 The relationship of immunosuppressive medications to levels of blood pressure, proteinuria, progression of CKD, and clinical CVD also needs to be studied. For example, the use of calcineurin-inhibitors impacts the development and severity of posttransplantation hypertension. The effect of antihypertensive agents may be different depending on the immunosuppressive regimen. When using protocols that avoid or minimize exposure to calcineurin inhibitors, the value of dihydropyridine calcium-channel blockers may not be as important. Finally, ambulatory blood pressure monitoring should be studied in this population since a disturbed day-night blood pressure rhythm is present and office blood pressure may not accurately reflect the burden of hypertension and its relationship to progression of kidney disease or CVD.504