Nondiabetic kidney diseases include glomerular diseases other than diabetes, vascular diseases other than renal artery disease, tubulointerstitial diseases, and cystic disease. Among these diseases, the level of proteinuria is useful for diagnosis and prognosis. Glomerular diseases are characterized by higher levels of proteinuria than other diseases. Higher levels of proteinuria are associated with faster progression of kidney disease and increased risk of CVD.
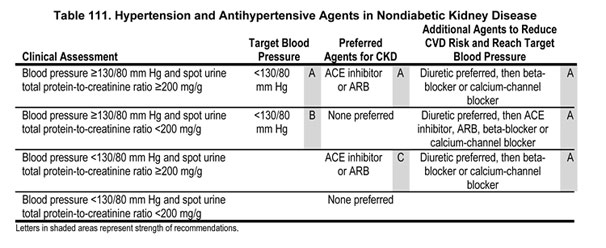
9.1 Target blood pressure in nondiabetic kidney disease should be <130/80 mm Hg (Guideline 7) (Table 111).
9.2 Patients with nondiabetic kidney disease and spot urine total protein to creatinine ratio ≥200 mg/g, with or without hypertension, should be treated with an ACE inhibitor or ARB (Table 111).
The term nondiabetic kidney disease encompasses a wide array of diseases which are often grouped together in epidemiological and controlled trials.442 Quantification of proteinuria is essential to the evaluation of nondiabetic kidney disease because of its important diagnostic, prognostic, and therapeutic implications.1,128 Glomerular diseases characteristically are accompanied by the early onset of higher levels of proteinuria. Lower levels of proteinuria are seen with vascular, tubulointerstitial, and cystic diseases. Proteinuria is also an important prognostic factor and is associated with more rapid progression of kidney disease and greater risk of CVD. The level of proteinuria also has therapeutic implications, which are addressed below.
Definitions
Clinical features of nondiabetic kidney disease, by stage, are shown in Table 112. For this guideline, we included studies of CKD Stages 1-4 due to "predominantly" nondiabetic kidney disease (fewer than approximately 15% of patients with diabetic kidney disease). Specifically, we included glomerular diseases other than diabetes, vascular diseases other than renal artery disease, tubulointerstitial diseases, and cystic disease, either separately or mixed. Studies of kidney transplant recipients were excluded. For evaluation of CVD outcomes, we extrapolated from studies in the general population (Guideline 7). We also included studies of CVD in individuals with predominantly nondiabetic kidney disease.
For this guideline, ACE inhibitors are compared to other classes of antihypertensive agents. There are few studies of ARBs in nondiabetic kidney disease. In these studies, diuretics were frequently used as an additional antihypertensive agent to achieve blood pressure control. In addition, some data are provided comparing other classes of antihypertensive agents.
Entries in summary tables comparing antihypertensive agents are grouped first by agent (ACE inhibitors first), then by control group medications (placebo then active control groups), then by methodological quality (highest first), then by applicability (widest first), and then by study size (largest first). Entries in summary tables comparing blood pressure targets are grouped by methodological quality (highest first), then by applicability (widest first), and then by study size (largest first).
Most patients with nondiabetic kidney disease are hypertensive (Strong). The prevalence of hypertension in clinical trials has been very high. In the ACE Inhibition in Progressive Renal Insufficiency (AIPRI) Study, the prevalence of hypertension was 92% and in the Ramipril Efficacy in Nephropathy (REIN) Study it was 84%. The prevalence of hypertension in the MDRD Study for types of nondiabetic kidney disease is shown in Table 113.21
Higher levels of blood pressure are associated with more rapid progression of nondiabetic kidney disease (Strong). A number of prospective studies show a strong relationship between a higher level of blood pressure and an increased risk of kidney failure and worsening kidney function in nondiabetic individuals with CKD and in the general population.93,443
Several studies suggest that higher SBP is more important than higher DBP or high pulse pressure for kidney disease progression.405,444,445
Multiple antihypertensive agents are usually required to reach target blood pressure (Strong). As demonstrated in Table 114, at least two to three antihypertensive agents were required to achieve blood pressure control in controlled trials of nondiabetic CKD.
ACE inhibitors are more effective than other antihypertensive agents in slowing the progression of most nondiabetic kidney diseases (Strong). The beneficial effect is greater in patients with higher levels of proteinuria (Strong). Several large, randomized trials of participants with nondiabetic kidney disease determined that regimens including ACE inhibitors are more effective in reducing the occurrence of kidney endpoints compared to regimens not including ACE inhibitors (Table 115). Most early studies were relatively small, less than 100 patients, and reported variable efficacy based on surrogate endpoints (such as doubling of serum creatinine or decrement in proteinuria). Two of these studies, the ACE Inhibition in Progressive Renal Insufficiency (AIPRI) Study and Ramipril Efficacy in Nephropathy (REIN) Study, were large, multicenter studies that showed conclusive results. However, only the REIN Study showed a beneficial effect on ACE inhibitors in reducing the incidence of kidney failure. Some studies suggested that the beneficial effect of ACE inhibitors was mediated by factors in addition to their antihypertensive effect. Most of the trials enrolled patients with a variety of nondiabetic kidney diseases, and subgroup analyses from some trials suggested a greater beneficial effect in patients with glomerular diseases, as compared with nonglomerular diseases (Table 116).
Supporting these conclusions, a meta-analysis by the ACE Inhibition of Progressive Renal Disease (AIPRD) Study Group of patient-level data on 1,860 nondiabetic patients enrolled in 11 RCTs of various ACE inhibitors (including many of the studies in Table 115) found that the ACE inhibitor group had better blood pressure control, lower urine protein excretion, and an approximately 30% reduction in the risk of kidney failure, as well as a reduction in the combined endpoint of doubling of serum creatinine or onset of kidney failure (Fig 44).458 Moreover, an incrementally greater beneficial effect was seen with greater degrees of proteinuria, with a threshold value of approximately 0.5 g/d (Fig 45). The larger beneficial effect of ACE inhibitors in patients with proteinuria appeared to be due to their greater antiproteinuric effects in patients with greater proteinuria.459 The beneficial effects of ACE inhibitors to slow kidney disease progression appeared to be mediated by factors in addition to their effects on blood pressure and proteinuria.
Fig 44. ACE Inhibition in Progressive Renal Disease (AIPRD) Study Group Pooled Analysis. Effects on blood pressure (A), urinary protein excretion (B), survival without kidney failure (C), or the combined outcome of doubling of baseline serum creatinine or kidney failure. Patients taking ACE inhibitors (dotted line) and controls (solid line). Follow-up measurements were reported more often during the first 2 years and less often thereafter. Mean blood pressure and mean urine protein excretion during follow-up were defined as the mean of all available follow-up values for each patient. Change during follow-up was defined as the baseline value minus the mean follow-up value for each patient. The number of patients with follow-up data available for analysis of survival without kidney failure is shown below the x-axis of (C). Reprinted with permission.458
Fig 45. ACE Inhibition in Progressive Renal Disease (AIPRD) Study Group Pooled Analysis. Relationship of baseline urinary protein to risk for kidney failure (A) or combined outcome of doubling of baseline serum creatinine or kidney failure (B), or relative risk for these outcomes (C and D). Patients taking ACE inhibitors (squares) and controls (circles). The values above the graphs in (A) and (B) are the fractions of patients with event in the control group (upper row) and ACE inhibitor group (lower row). Relative risks were calculated form multivariable models controlling for significant baseline patient and study characteristics. The solid horizontal line at a relative risk of 1.0 in (C) and (D) indicates no difference between the ACE inhibitor and control groups; the solid and dotted curved lines represent point estimates and 95% CI for the relative risks. P values for tests for interactions between baseline urinary protein excretion and treatment were 0.03 and 0.001, respectively. Reprinted with permission.458
The beneficial effects of ACE inhibitors were also seen in the African-American Study of Kidney Disease and Hypertension (AASK), a randomized trial in patients with hypertensive nephrosclerosis. In this trial, participants were randomly assigned to initial treatment with an ACE inhibitor (ramipril), a beta-blocker (metoprolol), or a dihydropyridine calcium-channel blocker (amlodipine). No difference was observed between the drug groups for the primary outcome of rate of change of GFR. However, the ACE inhibitor was more effective than amlodipine and metoprolol in reducing the secondary composite kidney outcome (50% reduction of GFR, ESRD or death) (Fig 46).141 Since blood pressure control was similar among the three drug groups, the beneficial effects of ACE inhibitors were mediated by factors in addition to the effects on blood pressure. Furthermore, a larger beneficial effect was observed in patients with greater levels of proteinuria.
Fig 46. African-American Study of Kidney Disease and Hypertension (AASK). Clinical composite events include declining GFR, kidney failure or death. Ramipril versus metoprolol (RR = 0.78, P = 0.042; metoprolol versus amlodipine (RR = 0.80, P = 0.17); ramipril versus amlodipine (RR = 0.62, P = 0.004). RR computed after adjustment for baseline covariates. Reproduced with permission.454
Relatively few patients with polycystic kidney disease (PKD) were enrolled in these trials. In general, patients with PKD have low mean levels of proteinuria but have a fast mean decline in GFR. ACE inhibitors lower urine protein levels in PKD, but there are insufficient data to conclude whether ACE inhibitors slow the progression of PKD.
In contrast to these studies in nondiabetic kidney disease, a recent analysis of the large subgroup of nondiabetic individuals with estimated 60 mL/min/1.73 m2 enrolled in ALLHAT showed no beneficial effects of an ACE inhibitor (lisinopril) compared to a diuretic (chlorthalidone) or a dihydropyridine calcium-channel blocker (amlodipine) on decline in GFR or onset of kidney failure over a 4-year interval.293 However, as discussed in Guideline 7, there are a number of differences between ALLHAT and controlled trials of kidney disease progression. It is the opinion of the Work Group that the ALLHAT results do not rule out a beneficial effect of ACE inhibitors in nondiabetic kidney disease, particularly in patients with proteinuria. Instead, the Work Group concluded that ACE inhibitors should be used to delay the progression of most nondiabetic kidney diseases.
ARBs may be more effective than other antihypertensive agents in slowing the progression of nondiabetic kidney disease (Weak). The Work Group found no large, long-term, RCTs comparing antihypertensive regimens including ARBs to regimens without ARBs in nondiabetic kidney disease. However, in short-term studies using surrogate end-points, ARBs appear similar to ACE inhibitors in lowering blood pressure and reducing proteinuria.460-462 In addition, their efficacy in animal models of nondiabetic kidney disease appears similar to the efficacy of ACE inhibitors.463 Therefore, the Work Group concluded that ARBs are probably as effective in slowing the progression of nondiabetic kidney disease, and strongly recommended treatment with an ARB if an ACE inhibitor cannot be used.
ACE inhibitors and ARBs in combination may be more effective than either alone in slowing the progression of nondiabetic kidney disease (Weak). A recent trial reported that combination therapy with an ACE inhibitor and ARB more effectively retarded progression of nondiabetic kidney disease than therapy with either agent alone.464 It was the opinion of the Work Group that the impact of combined therapy needs to be confirmed in other studies with a larger sample size.
Diuretics may potentiate the beneficial effects of ACE inhibitors and ARBs in nondiabetic kidney disease (Moderately Strong). In most clinical studies, diuretics were used in addition to ACE inhibitors. In the AASK study, 74% of the ACE inhibitor group received furosemide. In contrast, use of diuretics was restricted in the ACE inhibitor group in ALLHAT. Potentially, the combination of agents that block the RAS and diuretics is more effective than either agent alone. As discussed earlier, most patients will require more than one antihypertensive agent to reach the target blood pressure. It is the opinion of the Work Group that most patients with nondiabetic kidney disease should be treated with a combination of ACE inhibitor and a diuretic to reach the target blood pressure.
ACE inhibitors, ARBs, and nondihydropyridine calcium-channel blockers have a greater antiproteinuric effect than other antihypertensive classes in nondiabetic kidney disease (Strong). Two meta-analyses have demonstrated a greater effect of ACE inhibitors compared to other classes of antihypertensive agents on reducing proteinuria in nondiabetic kidney disease (Figs 40 and 41). Other studies show a larger effect of ARBs compared to other classes. Antiproteinuric effects of antihypertensive agents may be additive in nondiabetic kidney disease. ACE inhibitors and ARBs can be used in combination, and combination is better than monotherapy with ACE inhibitor or angiotensin receptor antagonist at recommended dosage.465 As in diabetic kidney disease (Guideline 8), it was the opinion of the Work Group that it would be reasonable to add a nondihydropyridine calcium-channel blocker to an ACE inhibitor or ARB to slow the progression of nondiabetic kidney disease.
Dihydropyridine calcium-channel blockers are less effective than other agents in slowing the progression of nondiabetic kidney disease with proteinuria (Moderately Strong). The AASK Study was the first large study to examine the impact of a dihydropyridine calcium-channel blocker on nondiabetic kidney disease. As described earlier, this study examined the effects of an ACE inhibitor (ramipril), a dihdyropyridine calcium-channel blocker (amlodipine), and a beta-blocker (metoprolol). These were multidrug regimens with open-label, add-on drugs other than those in the three classes of study agents. When compared with amlodipine, both ramipril and metoprolol reduced the risk of kidney failure and of kidney failure and death combined (Fig 46). In addition, the rise in proteinuria was significantly higher in the amlodipine group than the other two drug groups.
In contrast, ALLHAT did not find a detrimental effect of amlodipine on the decline in GFR or onset of kidney failure compared to chlorthalidone or lisinopril. However, as discussed in Guideline 7, there are a number of differences between ALLHAT and studies of kidney disease progression. In particular, it is likely that nondiabetic participants in ALLHAT did not have substantial proteinuria. Thus it is possible that the discrepant results between AASK and ALLHAT reflect differing effects of dihydropyridine calcium-channel blockers on progression of kidney disease with and without proteinuria.
For these reasons, it was the opinion of the Work Group that dihydropyridine calcium-channel blockers should not be used in nondiabetic kidney disease with proteinuria in the absence of therapy with an ACE inhibitor or ARB. A dihydropyridine calcium-channel blocker could likely be used safely in combination with patients taking an ACE inhibitor or ARB. In patients without proteinuria, a dihydropyridine calcium-channel blocker is likely safe when used in combination with a diuretic or alone.
A SBP goal of <130 mm Hg is more effective in slowing the progression of nondiabetic kidney disease in patients with proteinuria (Strong). An even lower blood pressure goal may be more effective in patients with proteinuria >500 to 1,000 mg/g (Weak). The SBP goal recommended for CVD risk reduction (<130/80 mm Hg) (Guideline 7) corresponds to the achieved SBP in many of the studies reviewed in the summary table. The potential beneficial effect on kidney disease progression of a lower blood pressure goal has been investigated in two large controlled trials (Table 117). In the Modification of Diet in Renal Disease (MDRD) Study and AASK, patients were randomly assigned to a mean arterial pressure (MAP) goal of <92 mm Hg (corresponding to <125/75 mm Hg), compared to a MAP goal of <107 mm Hg (corresponding to <140/90 mm Hg). In the MDRD Study, a study of predominantly nondiabetic kidney disease of various causes, mean baseline proteinuria was 2.2 g/d. A beneficial effect of the lower blood pressure goal was observed in patients with higher rates of urinary protein excretion (Fig 47).466 The threshold level of proteinuria below which there was no substantial benefit was 0.5 to 1.0 g/d (Fig 48).467 In the AASK Study, participants had a mean baseline proteinuria of less than 1.0 g/d. There was no significant beneficial effect of the lower blood pressure goal (Fig 49).141 However, there was a trend favoring the lower blood pressure goal in participants with higher baseline proteinuria and an opposite trend in participants with little or no proteinuria.
Fig 47. Modification of Diet in Renal Disease (MDRD) Study. Effect of strict blood pressure control on GFR decline. For Study A (baseline GFR 25 to 55 mL/min/1.73 m2), estimated mean (ąSE) rates of decline in GFR from baseline to 3 years, based on a 2-slope model are shown. For Study B (baseline GFR 13 to 24 mL/min/1.73 m2), mean (ąSE) rates of decline in GFR are estimated from the 1-slope informative censoring model. Closed circles designate the usual blood pressure group; open circles designate the low blood pressure group. The number in parentheses in each column is the total number of patients in both blood pressure groups who had a least one follow-up measurement. Greater baseline proteinuria is associated with a steeper mean GFR decline and with a greater benefit form the low blood pressure goal (P = 0.02 in Study A; P = 0.01 in Study B). Reprinted with permission.467
Fig 48. Modification of Diet in Renal Disease (MDRD) Study. Relationship between mean arterial blood pressure and GFR decline. Mean GFR decline and achieved follow-up blood pressure in the MDRD Study A (patients with baseline GFR 25 to 55 mL/min/1.73 m2). Regression lines relating the estimated mean GFR decline over 3 years to mean follow-up MAP for groups of patients defined according to baseline proteinuria. Within each group, a 3-slope model was used with break points at 92 and 98 mm Hg. Reprinted with permission.467
Fig 49. African-American Study of Kidney Disease and Hypertension (AASK). Effect of strict blood pressure control on events. Clinical composite event defined as declining GFR, kidney failure, or death. Mean achieved blood pressures of 141/85 mm Hg in the usual group and 127/78 mm Hg in the low group. Relative risk of low versus usual goal = 0.98 (0.79 to 1.22), P = 0.85. Reprinted with permission.45
The AIPRD Study Group has also reported a meta-analysis of individual patient level data relating the level of SBP and proteinuria during follow-up to the risk of the combined endpoint of doubling of serum creatinine or onset of kidney failure.471 These results indicate that the optimal level of SBP to slow kidney disease progression was lower in patients with proteinuria (Fig 50). In patients with proteinuria ≥ 1.0 g/d, the optimal SBP was 110 to 129 mm Hg. In patients with proteinuria <1.0 g/d, there was no significant relationship between the level of SBP and risk of kidney disease progression. Irrespective of urine protein excretion, SBP below 110 mm Hg was associated with a higher risk of kidney disease progression (Fig 50). However, it is unclear whether this was the results of decrease kidney perfusion or related to independent factors such as more serious comorbidity in the individuals with lower blood pressure.
Fig 50. ACE Inhibition and Progessive Renal Disease (AIPRD) Study Group Pooled Analysis. Relative risk for kidney disease progression based on levels of SBP and urinary protein excretion during follow-up. Filled and open symbols show the relative risk for patients with current urine protein excretion ≥1.0 g/d (n = 9,336 visits, 223 events) and <1.0 g/d (n = 13,274 visits, 88 events), respectively. Reference group for each is defined at SBP 110 to 119 mm Hg. Results are from a single multivariable model including two levels for urine protein excretion, six levels for SBP and interaction of current SBP and current urine protein excretion. Covariates include assignment to ACE inhibitor versus control group, gender, age, baseline SBP, baseline diastolic blood pressure, baseline urine protein excretion, baseline serum creatinine concentration (<2.0 or ≥2.0 mg/dL), interaction of baseline serum creatinine and baseline urine protein excretion, interaction of baseline serum creatinine and current urine protein excretion, and study terms. Reprinted with permission.471
Overall, these studies provide strong evidence that the target SBP of <130/80 mm Hg recommended for CVD risk reduction also slows the progression of kidney disease with proteinuria. An even lower blood pressure goal can be considered for patients with proteinuria greater than approximately 1.0 g/d. NKF-K/DOQI CKD Guidelines recommend that proteinuria be quantitated based on spot urine measurements. Thus, for convenience, the Work Group recommends consideration of an even lower blood pressure goal for patients with a spot urine total protein to creatinine ratio >500 to 1,000 mg/g. The studies do not allow definition of a precise systolic blood pressure goal below 130 mm Hg. Clinicians should be cautious about lowering systolic blood pressure below 110 mm Hg. Patients treated with antihypertensive agents and SBP <120 mm Hg should be monitored more frequently (Guidelines 11 and 12).
Comparison With Other Guidelines
The elements of this guideline are similar to recently published guidelines which also emphasize the importance of blood pressure control and the protective effects of ACE inhibitors (CARI guidelines, British Renal Association, Veteran’s Health Administration, and Institute for Clinical System Improvement). Although previous guidelines have recommend use of ACE inhibitors only in patients with urine protein excretion >1 g/d, Guideline 9 reflects the recent findings of the AIPRD Study Group meta-analysis and AASK Study, suggesting a beneficial effect of ACE inhibitors in patients with a lower levels of proteinuria. The threshold value for spot urine total protein-to-creatinine of ≥200 mg/g selected by the Work Group is arbitrary, but supported by the evidence. JNC 7 recommends ACE inhibitors or ARBs in nondiabetic kidney disease without reference to the level of proteinuria. However, that recommendation is not consistent with the results of ALLHAT, so the Work Group elected to maintain the recommendation to base decision-making in nondiabetic kidney disease on the level of proteinuria.
Other guidelines have recommended a blood pressure goal of <125/70 to 75 mm Hg in patients with urine protein excretion >1 g/d. In most cases, those recommendations were made when the target blood pressure for patients with lower levels of proteinuria was <140/90 mm Hg. The Work Group concluded that the evidence supporting a blood pressure goal even lower than <130/80 mm Hg in patients with higher levels of proteinuria is weak, but that clinicians may wish to consider a lower blood pressure in selected patients.
Figure 51 and Table 118 summarize recommendations in nondiabetic kidney disease.
Fig 51. Hypertension and antihypertensive agents in nondiabetic kidney disease. Superscripts refer to items in Table 118.
One of the challenges in creating these guidelines is that nondiabetic kidney disease encompasses a diverse array of diseases. Differentiating the type of nondiabetic kidney disease is another challenge. As described earlier, urinary protein excretion, the urine sediment, and kidney imaging procedures may be useful (Table 112). However, there is large variation in urine protein excretion in types of kidney disease. Therefore, urine protein cut-off values to suggest types of nondiabetic kidney diseases are not precise, and urine sediment examination and kidney imaging may not be diagnostic. Another limitation in approaching nondiabetic kidney disease is that there are few large studies of a single type of nondiabetic kidney disease. Further modifications of these recommendations will require the development of more discriminating diagnostic techniques and large studies focusing on single types of nondiabetic CKD.
In the clinical studies that have been reviewed, participants with kidney disease required an average of three to four antihypertensive agents and frequent follow-up visits. Some challenges to the implementation of these guidelines include costs, side-effects, and the significant commitment required of both the patient and their provider.
Recommended areas for future research include:
In all studies, if feasible, it would be preferable to focus on specific types (diagnoses) of CKD due to causes other than diabetes.