The recommendation for protein intake in children with CKD has to consider maintenance of growth and an adequate nutritional status, but also the intrinsic link of DPI and phosphorus load. The growing evidence for a major impact of phosphorus overload on cardiovascular morbidity in children and adults with CKD provides a rationale to avoid excessive protein intake in this population. At a given level of quantitative protein intake, the phosphorus content and bioavailability of the protein sources, the quality of protein, and the metabolic environment are important additional factors to consider in the dietary protein prescription.
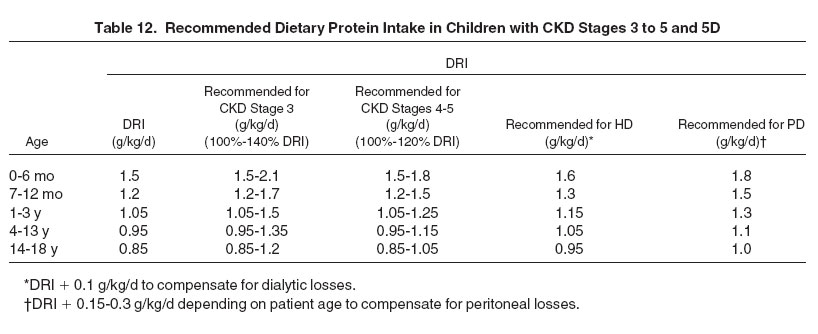
5.1 It is suggested to maintain dietary protein intake (DPI) at 100% to 140% of the DRI for ideal body weight in children with CKD stage 3 and at 100% to 120% of the DRI in children with CKD stages 4 to 5. (C)
5.2 In children with CKD stage 5D, it is suggested to maintain dietary protein intake at 100% of the DRI for ideal body weight plus an allowance for dialytic protein and amino acid losses. (C)
5.3 The use of protein supplements to augment inadequate oral and/or enteral protein intake should be considered when children with CKD stages 2 to 5 and 5D are unable to meet their protein requirements through food and fluids alone. (B)
5.1: It is suggested to maintain dietary protein intake at 100% to 140% of the DRI for ideal body weight in children with CKD stage 3 and at 100% to 120% of the DRI in children with CKD stages 4 to 5. (C)
Progressive CKD is generally associated with a reduction in spontaneous dietary intake of both protein and energy. In a study comparing 50 children with CKD stages 3 to 4 with healthy controls, protein intake was found to be 33% lower and energy intake was 10% lower in patients with CKD.255 However, whereas spontaneous energy intake tends to be critically low, eg, less than 80% to 85% of the RDA, DPI in those with CKD is far in excess of the average requirements, typically 150% to 200% of the RDA.9,255,256
The efficacy of low-protein diets in reducing the rate of CKD progression has been assessed in randomized prospective trials in both adult and pediatric patients. In the MDRD trial, no significant beneficial effect of decreasing DPI from 1.3 to either 0.58 or 0.3 g/kg/d, supplemented with essential keto acids, could be demonstrated; subtle signs of a suboptimal nutritional status were noted with these diets.257 In a pediatric trial involving 191 children with CKD stages 3 to 4, a reduction in protein intake aiming at 100% (0.8 to 1.1 g/kg ideal body weight [defined as the weight at the same percentile as the child's height percentile for the same age and sex]) and achieving 120% of the dietary intake recommended by WHO did not alter the rate of CKD progression compared with a cohort with ad libitum protein intake (mean, 181% of RDA).256,258 The reduction in protein intake, with maintenance of energy intake at greater than 80% of the RDA in both groups, did not affect statural growth, weight gain, body composition, or serum albumin levels within the observation period of 2 to 3 years.
Hence, although there is no evidence for a nephroprotective effect of dietary protein restriction, protein intake can be restricted safely to 0.8 to 1.1 g/kg/d in children with CKD. Because dietary protein restriction reduces the accumulation of nitrogenous waste products and facilitates lowering dietary phosphorus intake, it appears appropriate to gradually lower DPI toward 100% of the DRI in children advancing from CKD stage 3 to stage 5. This should delay the onset of signs and symptoms of uremia, although it should be noted that in the pediatric trial cited, the time of initiation of kidney replacement therapy was not delayed significantly in the low-protein cohort. Moreover, implementation and maintenance of a strict low-protein diet requires a major lifestyle change that may not be acceptable to many families. Hence, moderate protein restriction aiming at 100% to 140% of the DRI in CKD stage 3 and 100% to 120% of the DRI in CKD stages 4 to 5 may be a reasonable compromise in most cases (Table 12).
These protein recommendations refer to a stable child and assume that energy intake is adequate (ie, it meets 100% of estimated requirements). Inadequate caloric intake results in the inefficient use of dietary protein as a calorie source, with increased generation of urea. Ensuring caloric needs are met is an important step in assessing protein requirements and modifying protein intake.
Protein requirements may be increased in patients with proteinuria and during recovery from intercurrent illness. Modification of protein recommendations also may be necessary in obese or stunted children. Obese individuals have a greater percentage of body fat, which is much less metabolically active than lean mass. Therefore, it is believed that basing protein (and energy) requirements of obese individuals on their actual weight may overestimate requirements. Conversely, using ideal body weight for an obese person does not take into account the increase in body protein needed for structural support of extra fat tissue. Therefore, a common practice is to estimate protein requirements of obese individuals based on an "adjusted" weight (ie, adjusted weight = ideal weight for height + 25% × [actual weight − ideal weight], where 25% represents the percentage of body fat tissue that is metabolically active) rather than their actual body weight.259 This formula is based on physiological theory rather than scientific evidence. In young children (ie, age <3 years) or stunted children (ie, length- or height-for-age < −1.88 SDS), protein requirements initially should be estimated by using chronological age, but may be reestimated by using height age if there are indications of inadequate protein intake (see Recommendation 5.3).
5.2 In children with CKD stage 5D, it is suggested to maintain dietary protein intake at 100% of the DRI for ideal body weight plus an allowance for dialytic protein and amino acid losses. (C)
Our recommendations for DPI in dialyzed children differ from previous adult and pediatric guidelines based on several lines of reasoning.
First, the Food and Nutrition Board of the Institute of Medicine of the National Academy of Sciences in 2002 replaced the RDA of 1989 with DRI values for the intake of nutrients by Americans and Canadians. For protein, the DRI values are lower than the RDA across all age groups.175
Second, previous recommendations for dialyzed patients were based on the concept that in addition to replacements for dialytic amino acid and protein losses, at least 0.3 to 0.4 g/kg of dietary protein should be added to the intake recommended for healthy subjects.62 The evidence base for this notion is weak and primarily based on adult literature.
The widespread notion that dialysis induces generalized protein catabolism through generalized protein degradation resulting from cytokine release induced by exposure to bioincompatible membranes (in HD) or dialysis fluids (in PD) has not been universally confirmed by metabolic studies. Net protein "catabolism" seems to be limited to the dialytic removal of amino acids and/or protein and a slightly reduced protein synthesis during HD sessions. Whole-body protein breakdown is not increased.260
Observational studies showing a correlation between high protein intake and better outcomes in adult dialysis patients261,262 do not prove that a high-protein intake by itself stimulates tissue anabolism. Reviews of nitrogen-balance studies performed in adult dialysis patients with different protein intakes56,263-270 conclude that HD patients are in neutral nitrogen balance with a protein intake as low as 0.75 to 0.87 g/kg/d, and PD patients, with 0.9 to 1.0 g/kg/d. A single nitrogen-balance study has been performed in dialyzed children.152 In 31 pediatric patients receiving automated PD, the investigators observed a positive correlation between nitrogen balance and DPI and concluded that DPI should be at least 144% of RDA. However, nitrogen balance also positively correlated with total energy intake, and no multivariate analysis was performed to address whether energy intake, protein intake, or both were independent effectors of nitrogen balance.
A single randomized prospective study in adults271 and several trials in children have addressed the effect of selectively increasing amino acid supply in patients on PD therapy. Despite increases in amino acid and dietary protein intake, no significant beneficial effects on nutritional status and longitudinal growth in children were achieved by this intervention, whereas urea concentrations frequently increased.272-276 These results are compatible with the interpretation that it is not possible to induce tissue anabolism by selectively increasing protein and amino acid ingestion except in subjects with subnormal baseline protein intake. If more protein is ingested than needed for metabolic purposes, all the excess is oxidized and results in accumulation of nitrogenous-containing end products.
Third, although evidence for beneficial effects of a high DPI is lacking, there is growing concern that it may even be harmful to dialyzed children. In a DXA study of body composition in 20 children on long-term PD therapy and a mean DPI of 144% of the RDA, protein intake inversely correlated with bone mineral density, bone mineral content, and fat-free mass, and also with plasma bicarbonate level, suggesting that a high protein intake may cause tissue catabolism and bone loss through aggravating metabolic acidosis.277
Finally, the most convincing argument for limiting DPI in dialyzed children is derived from the solid evidence for a key etiologic role of dietary phosphorus load in the pathogenesis of dialysis-associated calcifying arteriopathy in pediatric and adult patients. Several studies of children and adults with childhood-onset CKD stage 5 have demonstrated correlations between serum phosphorus levels and cumulative phosphate-binder requirements and arteriopathy,278-282 which, in turn, is linked to the excessive cardiovascular mortality of patients with CKD.283,284
There is a nearly linear relationship between protein and phosphorus intake,285 which determines a frequent association of high protein in the diet with hyperphosphatemia.286 Whereas hyperphosphatemia is a powerful independent predictor of mortality on dialysis therapy,287 evidence for any benefit from high-protein diets is lacking.288 Hence, it appears mandatory to limit protein intake to the safe levels known to ensure adequate growth and nutrition in healthy children.
The adverse impact of hyperphosphatemia on cardiovascular, bone, and endocrine function in children with CKD mandates the preferential selection of protein sources that are relatively low in phosphorus. The lowest amount of phosphorus in proportion to the quantity and quality of protein comes from animal-flesh proteins (average, 11 mg of phosphorus per 1 g of protein), whereas eggs, dairy products, legumes, and lentils have higher phosphorus-protein ratios (average, 20 mg of phosphorus per 1 g of protein; Table 13). Complexity is added by the variable digestibility of dietary protein and bioavailability of dietary phosphorus. Protein digestibility from animal proteins is 95%, whereas protein digestibility from plant proteins (85%) and mixed meals (85% to 95%) is lower. Whereas phosphorus in animal meat is stored as organic phosphates in intracellular compartments that are easily hydrolyzed and readily absorbed, 75% of phosphorus in plants is in the form of phytic acid. Because humans do not express the degrading enzyme phytase, the bioavailability of phosphorus from plant-derived food is very low. Phosphorus availability from animal products is greater than 70%, whereas availability from plant products (50%) and mixed meals (50% to 70%) is lower. Hence, despite their higher specific phosphorus content, some plant sources of protein may actually result in a lower rate of phosphorus uptake per mass of protein than meat-based foods (see Appendix 3, Table 35).289 If healthy humans are administered an equivalent amount of either animal or plant protein, urinary phosphorus excretion is higher with the meat-based diet.290 Moreover, meat products are frequently "enhanced" by the addition of phosphate salts; these additions may markedly increase the total phosphorus load. Hence, a mixed composition of dietary protein with a strong contribution of vegetable protein rich in phytic acid should be encouraged.
Although dialyzed children require larger amounts of protein per unit of body weight than adults to grow in size and lean body mass, this demand is fully accounted for by the age-adjusted pediatric DRI. Hence, the only additional dietary protein requirement justified by evidence is the replacement of dialytic nitrogen losses. In those on long-term PD therapy, daily peritoneal protein losses decrease with age across childhood from an average of 0.28 g/kg in the first year of life to less than 0.1 g/kg in adolescents.292 Peritoneal amino acid losses add approximately one-third to the nitrogen lost with protein, resulting in an total additional dietary protein requirement ranging from 0.15 to 0.35 mg/kg, depending on patient age (see Table 12).
Peritoneal permeability for protein shows large interindividual variation, but appears to be relatively constant within subjects. Transperitoneal protein transport correlated with small-molecule transport rates; the peritoneal transporter status as assessed by using the PET provides some indication of the level of peritoneal protein losses. High peritoneal transporters tend to have low serum albumin levels; these patients may be at need for increased dietary protein supply. Because dialytic protein concentrations can be measured easily, consideration should be given to regular monitoring of peritoneal protein excretion and individual adaptation of the dietary protein prescription according to actual peritoneal losses.
Amino acid and protein losses during HD vary according to dialyzer membrane characteristics and reuse. Losses have not been quantified in children. In adults, an average of 8 to 10 g of amino acids and less than 1 to 3 g of protein are lost per HD session.288,293,293a,293b On the basis of 3 HD sessions per week for a 70 kg adult, this equates to 0.08 g/kg/day.† Assuming that dialytic amino acid losses are in linear relationship to urea kinetics, children can be expected to have similar or slightly higher amino acid losses than adults. An added DPI of 0.1 g/kg/d should be appropriate to compensate for pediatric hemodialytic losses (see Table 12). Under all conditions, at least 50% of dietary protein intake should be of high biological value‡ to protect body protein and mimimize urea generation.
In patients undergoing intensified HD modalities, in particular, extended nocturnal HD, the removal of nitrogenous waste products and phosphorus is almost doubled, frequently resulting in a need for phosphorus substitution.294 Appetite and spontaneous dietary energy and protein intake reportedly increase in these patients. The excellent nitrogen and phosphorus clearances achieved with intensified treatment schedules and the concomitantly increased amino acid losses permit and require liberalization of DPI.
These recommendations for DPI refer to dialyzed children in stable clinical condition. Protein requirements may be increased in patients with proteinuria, during and after peritonitis episodes, and during recovery from intercurrent illness.
5.3: The use of protein supplements to augment inadequate oral and/or enteral protein intake should be considered when children with CKD stages 2 to 5 and 5D are unable to meet their protein requirements through food and fluids alone. (B)
Occasionally, protein intake may be inadequate in children with CKD because of anorexia, chewing problems, or the need for very stringent phosphorus restriction. Suggested signs of inadequate protein intake include abnormally low serum urea levels, an undesirable downward trend in nPCR for adolescents on HD therapy (see Recommendation 1, nPCR), and/or documentation of low protein intake by using food records, food questionnaires, or diet recall. Powdered protein modules (Appendix 3; Table 36) can be added to expressed breast milk, infant formula, beverages, pureed foods, or other moist foods to boost their protein content, and minced or chopped meat, chicken, fish, egg, tofu, or skim milk powder can be added to soups, pasta, or casseroles. Liquid protein-rich renal supplements (Appendix 3) can also be used orally or enterally to boost protein intake.
The CARI CKD Guidelines recommend that children have a protein intake equivalent to or greater than those recommended by the Food and Agriculture Organization, WHO, and United Nations University for healthy children.
† (13 g AA and protein ×3 sessions)÷ 7 days per week ÷70 kg = 0.08 g/kg/d.
‡protein containing the 9 essential amino acids in a proportion similar to that required by humans has high biological value. When one or more essential amino acids are scarce, the protein is said to have low biological value. Animal sources of protein (eg, meat, poultry, fish, eggs, milk, cheese, yogurt) provide high biological value protein. Protein found in plants, legumes, grains, nuts, seeds and vegetables are of low biological value.