The management of oral and/or enteral calcium intake in children with CKD is a challenging problem for physicians and dietitians. Whereas insufficient calcium supply may cause deficient mineralization of the skeleton, calcium overload may be associated with severe vascular morbidity.
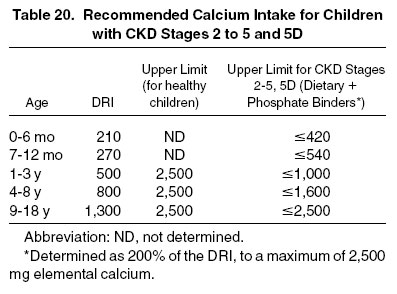
7.1.1 In children with CKD stages 2 to 5 and 5D, it is suggested that the total oral and/or enteral calcium intake from nutritional sources and phosphate binders be in the range of 100% to 200% of the DRI for calcium for age. (C)
Adequate dietary calcium intake during childhood is necessary for skeletal development, including acquisition of an optimal peak bone mass during puberty.355 Both insufficient and excessive oral and/or enteral calcium supply may occur in children with CKD. Intestinal calcium absorption is increasingly impaired in those with CKD as endogenous production of calcitriol (1,25-dihydroxyvitamin D; 1,25[OH]2D) decreases, but is readily stimulated by vitamin D therapy. Spontaneous calcium intake frequently is insufficient in adolescent patients in whom acceptance of high-calcium foods is limited and in children on phosphorus-restricted diets. The homeostatic mechanisms for regulating calcium balance are impaired most severely in children with CKD stage 5 and on dialysis therapy. Calcium absorption cannot be adjusted because of the kidney's inability to produce 1,25(OH)2D. Also, vitamin D receptor expression may be reduced.
However, therapy with high doses of active vitamin D sterols (eg, calcitriol, alfacalcidol) may boost intestinal calcium absorption. Oral and/or enteral treatment with calcium-containing phosphate binders and absorption from dialysis fluids with supraphysiological calcium content markedly enhance the calcium load. Increasing evidence suggests that the resulting strongly positive calcium balance is a major contributor to soft-tissue calcifications. Although it is impossible to accurately assess the actual absorption of calcium derived from diet and binders in this setting, it appears reasonable to limit total oral and/or enteral calcium ingestion.
Intake of 100% of the DRI for calcium is a reasonable starting point for children with CKD (Table 20). Although the safe limit of dietary calcium intake in children of different ages has not been defined by study evidence, it appears logical to scale maximal calcium intake relative to the age-specific DRI. The safe UL of dietary calcium intake in healthy individuals older than 1 year is 2,500 mg/d. For adults and children 9 years and older, this is approximately 2 times the DRI.
A number of measures are effective to improve low oral and/or enteral calcium intake and absorption: increased consumption of calcium-rich and/or calcium-fortified foods or tube feedings, supplementation with calcium-containing pharmacological agents between meals or bolus tube feedings, use of calcium-containing phosphorus binders for managing hyperphosphatemia, and supplementation with vitamin D.
If spontaneous intestinal calcium absorption is low, as typically observed in early stages of CKD, vitamin D should be supplemented to augment plasma 1,25(OH)2D synthesis and maximize calcium absorption.
If plasma calcium levels and urinary calcium excretion remain low and dietary assessment suggests inadequate calcium intake, consumption of foods with high endogenous calcium content (eg, milk, yogurt, cheese, Chinese cabbage, kale, and broccoli) and calcium-fortified food products should be encouraged. The bioavailability of calcium from milk and dairy products generally is high; however, the high phosphorus content of these products must be considered in children who require dietary phosphorus restriction. Some foods high in phytates, such as bran cereal, may have poor bioavailability of calcium.356-358 Fortified products seem to provide calcium bioavailability comparable to milk.359-361
If dietary intake alone does not meet the DRI, use of oral and/or enteral calcium supplements should be considered (Table 21). Salts of calcium—gluconate (9% elemental calcium), lactate (13% elemental calcium), acetate (25% elemental calcium), or carbonate (40% elemental calcium)—are usually well tolerated by children of all ages. Calcium-containing phosphate binders can be applied easily and effectively in infants. Conversely, calcium chloride should be avoided as a supplement in patients with CKD due to the possible development of metabolic acidosis. Calcium citrate should not be given because citrate augments aluminum absorption.362 Maximal absorption of calcium supplements is achieved when calcium salts are taken between meals and separate from iron supplements.363,364
As CKD progresses, increasing phosphate retention creates the need for oral and/or enteral phosphate-binder therapy. Calcium carbonate and calcium acetate are effective phosphate binders in children and should be used as first-choice therapy in patients with low dietary calcium intake.365-370 Calcium carbonate and calcium acetate easily can be crushed, dissolved in formula milk, and administered through enteral tubes. However, hypercalcemic episodes occur in approximately 25% of patients, depending on the type and dose of the calcium-containing binder and the coadministration of active vitamin D sterols (eg, calcitriol and alfacalcidol). Calcium acetate has a higher specific phosphorus-binding efficacy than calcium carbonate371 and causes fewer hypercalcemic episodes than calcium carbonate at a given phosphate-binder dose.372-374 Hence, calcium carbonate should be preferred in children with insufficient dietary calcium intake and no need for active vitamin D therapy, whereas calcium acetate is the preferable phosphate binder in children considered at moderate risk of calcium overload. In contrast to the use of calcium salts as supplements, calcium-containing phosphate binders should be taken with meals to obtain maximal phosphorus-binding efficacy and minimal intestinal absorption of free calcium. For calcium acetate, fecal excretion of phosphate has been shown to be higher when the phosphate binder is given with meals.375
The use of any calcium-containing phosphate binder should be limited by the maximally acceptable total oral and enteral calcium intake. For example, in a dialyzed 8-year-old with a typical spontaneous dietary calcium intake of 700 mg/d, a maximum of 900 mg of elemental calcium ingested as phosphate binders should be administered to stay within the recommended maximal total calcium intake of 1,600 mg (200% of the DRI). This would correspond to a prescription of 4 to 5 tablets containing 500 mg of calcium carbonate (200 mg of elemental calcium) or 5 tablets containing 667 mg of calcium acetate (167 mg of elemental calcium) per day. If dietary calcium intake is higher, calcium-containing phosphate-binder intake and/or dialysate calcium concentration need to be reduced, and the use of calcium-free phosphate binders should be considered. In a 1-year-old anuric child with an upper limit of 750 mg/d of calcium intake, a maximum of 875 mg of calcium carbonate (ie, 350 mg of elemental calcium) per day would be acceptable if dietary calcium intake is 400 mg.
These model calculations should be viewed as a general principle of dietary calcium prescription and may not always be applicable in clinical practice. Also, they do not consider confounding factors, such as treatment with active vitamin D sterols, which has been found to increase calcium absorption (reported to be 35% to 40% in those with CKD377) by 30%.378 The dosage of calcium-based phosphate binders should be reduced in dialysis patients with low PTH levels because these patients commonly have low-turnover bone disease with a reduced capacity of the bone to incorporate a calcium load.379
To avoid the critical accumulation of calcium, oligoanuric children on dialysis therapy may require a further reduction in total oral and enteral calcium intake from nutritional sources and phosphate binders. In those with CKD stage 5, urinary calcium excretion—the major physiological elimination pathway—is severely impaired or absent. An anuric child receiving HD or PD with a neutral dialysate calcium concentration is incapable of disposing of any calcium exceeding the amounts required for bone formation by any mechanism other than soft-tissue precipitation. Hence, the upper limit of dietary calcium intake considered safe in healthy subjects may not be applicable to oligoanuric patients. In these children, further limitation of oral and enteral calcium intake from both dietary sources and calcium-containing phosphate binders should be considered, although evidence to support this further restriction is not yet available. Modification to decrease the calcium concentration in the dialysate is an additional therapeutic option to be considered in both HD and PD patients. Calcium balance during PD usually is negative with the use of 2.5 mEq/L calcium dialysate and positive with 3.0 to 3.5 mEq/L calcium dialysate.380-384 Calcium balance during HD may be neutral or negative with the use of a 2.5-mEq/L calcium dialysate.385,386 Dietary and pharmacological interventions should aim at avoiding both hypo- and hypercalcemic episodes.
These recommendations are in agreement with the K/DOQI Clinical Practice Guidelines for Bone Metabolism and Disease in Children with Chronic Kidney Disease in limiting total oral and enteral calcium intake to 200% or less of the DRI. These guidelines differ in that the pediatric K/DOQI Bone Metabolism and Disease guidelines are more liberal and allow up to 2 times the DRI for elemental calcium by calcium-based phosphate binders and a total intake of elemental calcium of up to 2,500 mg/d, regardless of age.
7.2: Vitamin D
Recent clinical evidence suggests a high prevalence of vitamin D insufficiency in children and adults with CKD.
7.2.1 In children with CKD stages 2 to 5 and 5D, it is suggested that serum 25-hydroxyvitamin D levels be measured once per year. (C)
7.2.2 If the serum level of 25-hydroxyvitamin D is less than 30 ng/mL (75 nmol/L), supplementation with vitamin D2 (ergocalciferol) or vitamin D3 (cholecalciferol) is suggested. (C)
7.2.3 In the repletion phase, it is suggested that serum levels of corrected total calcium and phosphorus be measured at 1 month following initiation or change in dose of vitamin D and at least every 3 months thereafter. (C)
7.2.4 When patients are replete with vitamin D, it is suggested to supplement vitamin D continuously and to monitor serum levels of 25-hydroxyvitamin D yearly. (C)
A decrease in serum calcidol (25-hydroxyvitamin D; 25[OH]D), the substrate for renal synthesis of 1,25(OH)2D, induces secondary hyperparathyroidism in individuals with normal kidney function387,388 and may aggravate secondary hyperparathyroidism in patients with CKD.389,390 The critical lower limit of the serum vitamin D concentration is not well defined. Serum concentrations show considerable seasonal and regional variation. Although severe manifestations of vitamin D deficiency, such as osteomalacia and hypocalcemia, are seen only with 25(OH)D concentrations less than 5 ng/mL (<12 nmol/L), levels less than 30 ng/mL (75 nmol/L) are suggestive of vitamin D "insufficiency" as manifested by hyperparathyroidism and increased risk of bone demineralization and hip fractures.391,392 Supplementation with vitamin D, 800 IU/d, along with a modest dietary calcium supplement, reduced the hip fracture rate by 43% in a double-blinded placebo-controlled trial in elderly women.393
Vitamin D insufficiency is observed in a large proportion (typically 80% to 90%) of patients with CKD.394,395 In a population-based study of patients hospitalized in New England, CKD was a major risk factor for low serum 25(OH)D levels.396 Vitamin D insufficiency may be more relevant in those with CKD than in healthy individuals because, in contrast to healthy subjects in whom 25(OH)D is not rate limiting for calcitriol synthesis,397 1,25(OH)2D levels correlated with 25(OH)D levels in patients with CKD.394,395 This probably is explained by impaired compensatory upregulation of renal 1-α-hydroxylase and an increased contribution of strictly substrate-dependent extrarenal calcitriol synthesis in patients with impaired kidney function.398,399
Reasons for the high prevalence of low vitamin D levels in patients with CKD include their sedentary lifestyle with reduced exposure to sunlight, limited ingestion of foods rich in vitamin D (cod liver oil, fish, liver, egg yolk, fortified milk, and fortified margarine), reduced endogenous synthesis of vitamin D3 in the skin in patients with uremia,287 and urinary losses of 25(OH)D and vitamin D–binding protein in nephrotic patients.400
Even in patients with CKD stage 5D with little or no residual renal 1-α-hydroxylase activity, vitamin D deficiency is associated with more marked secondary hyperparathyroidism.401 In anephric individuals, high doses of ergocalciferol (D2) or alfacalcidol (25[OH]D) can increase serum calcitriol levels, pointing to a significant role of extrarenal 1-α-hydroxylase activity.402-404 However, the role of 25(OH)D deficiency and its correction in patients on maintenance dialysis therapy is controversial because the ability to generate adequate levels of 1,25(OH)2D is markedly reduced or absent. However, 25(OH)D has been claimed to exert specific effects on cell metabolism. 25(OH)D, but not 1,25(OH)2D, improved muscular function and phosphate content.405
In patients with CKD, nutritional vitamin D deficiency and insufficiency can be prevented or corrected by supplementation with vitamin D3 (cholecalciferol) or vitamin D2 (ergocalciferol). Cholecalciferol appears to have higher bioefficacy than ergocalciferol, although long-term comparative trials are lacking in humans.406,407 The DRI for prevention of vitamin D deficiency in children and adolescents is 200 IU.376 This value, published more than a decade ago, is 50% lower than the RDA that it replaced and, given increasing reports of vitamin D insufficiency in the general public, is controversial. The required daily vitamin D intake for patients of any age with CKD is unknown. In individuals with normal kidney function, the recommended upper limit of vitamin D is 1,000 IU/d in neonates and infants younger than 12 months and 2,000 IU/d for all other ages.376 The equivalent of this dose can be achieved by administering 1 capsule (50,000 IU) once a month.408 Daily doses of 10,000 IU of ergocalciferol have been administered in adult patients with advanced CKD for periods longer than 1 year with no evidence of vitamin D overload or renal toxicity.409,410 Whereas signs of vitamin D intoxication would be the exception at doses recommended in this guideline, the development of hypercalcemia would be evidence of excessive dosing.
We recommend treating vitamin D deficiency and insufficiency, with the specific dosing regimen dependent on the severity of the disorder (Table 22). Smaller doses of vitamin D probably are sufficient in children younger than 1 year. When repletion (ie, serum 25[OH]D ≥ 30 ng/mL) has been accomplished, vitamin D homeostasis should be maintained by once-daily administration of 200 to 1,000 IU.
Calcitriol, alfacalcidol, or other synthetic active vitamin D analogs (eg, doxercalciferol and paracalcitol) should not be used to treat 25(OH)D deficiency.
Our recommendations are in line with the K/DOQI Clinical Practice Guidelines for Bone Metabolism and Disease in Children with CKD.
The doses of ergocalciferol or cholecalciferol required to correct vitamin D insufficiency and to maintain normal vitamin D plasma levels have not been established in children with different stages of CKD.
7.3: Phosphorus
7.3.1 In children with CKD stages 3 to 5 and 5D, reducing dietary phosphorus intake to 100% of the DRI for age is suggested when the serum PTH concentration is above the target range for CKD stage and the serum phosphorus concentration is within the normal reference range for age. (C)
7.3.2 In children with CKD stages 3 to 5 and 5D, reducing dietary phosphorus intake to 80% of the DRI for age is suggested when the serum PTH concentration is above the target range for CKD stage and the serum phosphorus concentration exceeds the normal reference range for age. (C)
7.3.3 After initiation of dietary phosphorus restriction, it is suggested that serum phosphorus concentration be monitored at least every 3 months in children with CKD stages 3 to 4 and monthly in children with CKD stage 5 and 5D. (C) In all CKD stages, it is suggested to avoid serum phosphorus concentrations both above and below the normal reference range for age. (C)
Epidemiological studies of adult patients with CKD have demonstrated a positive association, albeit not a causal link, between hyperphosphatemia and morbidity and mortality independent of CKD stage. Although the benefits of lowering serum phosphorus level on patient-level clinical outcomes have not been demonstrated in prospective interventional studies, it is generally accepted and biologically plausible that increased serum phosphorus levels be avoided in patients with CKD stages 3 to 5 and 5D in an effort to control CKD-associated bone disease and CVD. Associations between hyperphosphatemia and CKD-associated vasculopathy have also been observed in children with CKD stage 5.282,411
Although serum phosphorus levels usually are not increased in the early stages of progressive CKD,363,412-414 the dietary phosphorus load is an important determinant of the severity of hyperparathyroidism, even in those with mild renal insufficiency. In children and adults with CKD stage 3, dietary phosphorus restriction decreases increased PTH levels and increases 1,25(OH)2D production, whereas dietary phosphorus intakes approximately twice the DRI for age aggravate hyperparathyroidism despite little or no change in serum phosphorus levels.413,415,416 Also, bone biopsy studies showed marked improvement in bone resorption and defects in bone mineralization by using dietary phosphate restriction.415 In 4 studies in children, dietary phosphate restriction did not lead to impaired statural growth.256,417-419 Studies in adult and pediatric patients provided no evidence for any adverse effect of dietary phosphate restriction on nutritional status.256,257,420-423 However, severe restriction of dietary phosphorus in children with moderate and severe CKD leading to subnormal serum phosphorus levels was associated with histological findings of worsening osteomalacia.415
Hence, a solid body of evidence suggests that moderate dietary phosphate restriction is beneficial with respect to the prevention and treatment of hyperparathyroidism and safe with respect to growth, nutrition, and bone mineralization. We recommend limiting dietary phosphorus intake to 100% of the DRI (Table 23) in normophosphatemic patients (using/not using phosphorus-lowering medications) if serum PTH concentration exceeds the target range (Table 24). Although similar PTH target ranges have been recommended by 2 Expert Work Groups,121,424 the optimal range is controversial and may be lower than previously believed.425 In CKD stages 4 and 5, when serum phosphorus levels increase to greater than the target normal range for age (Table 25) and hyperparathyroidism is already established, phosphorus restriction to approximately 80% of the DRI is recommended.
Higher physiological serum concentrations of calcium and phosphorus are observed in healthy infants and young children, presumably reflecting the increased requirements of these minerals by the rapidly growing skeleton. Rickets due to phosphorus deficiency occurs in preterm infants fed insufficient amounts of phosphorus and in infants and children with hypophosphatemia due to inherited disorders of renal phosphate transport.426 Hence, when dietary phosphorus is restricted to control hyperphosphatemia and secondary hyperparathyroidism in children with CKD, subnormal serum phosphorus values should be avoided (Table 25).
The dietary prescription should aim at minimizing phosphate intake while ensuring an adequate protein intake. To achieve this aim, protein sources with low specific phosphorus content should be prescribed (see Table 13, Recommendation 5). Most food sources exhibit good phosphate bioavailability with the exception of plant seeds (beans, peas, cereals, and nuts) that contain phosphate in phytic acid.
Milk and dairy products are a major source of dietary phosphorus. In young infants with CKD, phosphorus control can be achieved easily by using formulas with a low phosphorus content. It usually is feasible, and common clinical practice, to continue oral and/or enteral use of a low-phosphorus formula and delay the introduction of phosphorus-rich cow's milk until the age of 18 to 36 months.
Dietary phosphate restriction can be hindered by the inadvertent consumption of food containing phosphate additives, which can increase phosphorus intake up to 2-fold compared with unprocessed foods. This is a particular problem in patients with CKD who rely heavily on processed foods.427,428
Unfortunately, most available nutrient databases do not consider the impact of additives on total phosphorus content of foods. An exception is the USDA National Nutrient Database for Standard Reference, which lists more than 60 phosphate-containing food additives (www.ars.usda.gov/Main/site_main.htm?modecode=12-35-45-00; last accessed October 23, 2008).
The aspects mentioned illustrate that dietary modification of phosphorus intake is a complex and challenging task. Multiple pitfalls, including nonadherence in older children and adolescents, may result in inefficient lowering of phosphorus intake; conversely, overrestriction may lead to signs of phosphate deficiency, particularly in young infants. Hence, involvement of an experienced pediatric dietitian is key to phosphorus management in children with CKD.
A recent randomized clinical trial assessed the efficacy of a low-phosphorus diet compared with additional treatment with different phosphate binders in adults with CKD stages 3 to 5. Coronary calcification increased in patients on the low-phosphorus diet alone, to a lesser extent in calcium carbonate-treated patients, and not at all in sevelamer-treated patients.429 Notably, urinary phosphorus excretion did not decrease by the institution of the low-phosphorus diet alone and increased by 50% during the 2-year follow-up. These results highlight the difficulty of implementing and maintaining a phosphorus-restricted diet in clinical practice. Hence, dietary phosphate restriction should be considered an important, but not solitary, component in the management of uremic bone and vascular disease in association with vitamin D and phosphate-binder therapy and dialytic removal.
The link between hyperphosphatemia and patient mortality observed in adult studies287,430-432 and the associations between serum phosphorus level and surrogate markers of vascular morbidity in adult and pediatric patients with CKD282,433,434 provide a rationale to lower serum phosphorus levels pharmacologically if dietary phosphorus restriction is insufficient to maintain normophosphatemia. The current goal to target normal phosphorus levels is different from the allowance for slightly higher phosphorus values within the K/DOQI Pediatric Bone Guidelines.121 Oral and enteral phosphate binders are effective in lowering serum phosphorus concentrations in children with CKD.365-371 It should be noted that the association between bone and mineral metabolism disorders and cardiovascular risk and mortality are largely reported from either in vitro or retrospective cohort studies, which can prove association, but not cause and effect.
If total intestinal calcium load becomes excessive or hypercalcemia exists with the use of calcium-containing phosphate binders, these should be reduced in dose or replaced by calcium- and aluminium-free phosphate binders. The only calcium- and aluminum-free phosphate binder with proven efficacy and safety in children is sevelamer, which has been assessed in 2 randomized controlled clinical trials studying a total of 47 children. In 1 study, 29 hemodialyzed children were assigned to either sevelamer or calcium carbonate, and either calcitriol or doxercalciferol, as well. Although serum phosphorus levels were equally well controlled in the sevelamer and calcium-carbonate arms at the end of the 8-month study period, serum calcium and calcium-phosphorus ion product levels were significantly higher and hypercalcemia episodes were more frequent in the calcium-carbonate group, with no significant difference in serum PTH levels.435 The second trial used a crossover design to compare sevelamer with calcium acetate in 18 children with CKD stages 3 to 4 or 5D during 8-week observation periods. Phosphorus and PTH control were similar with both treatments, whereas hypercalcemia occurred more frequently with calcium acetate. A decrease in LDL cholesterol levels by 34% and a greater incidence of metabolic acidosis were observed with sevelamer.436
Sevelamer is a resin that, in aqueous solution, attains a gel-like consistency and cannot be applied through feeding tubes without a high risk of tube blockage. However, it is possible to pretreat breast milk,437 infant formula, and cow's milk438 by dissolving sevelamer, waiting for precipitation, decanting, and feeding the supernatant of the processed fluid. This maneuver reduces phosphorus content by 80% to 90%.
Larger comparative trials in adults consistently observed lower serum calcium and higher PTH levels with sevelamer than with calcium-containing phosphate binders.256,422,426-429,435-437,439-441 In adult patients with CKD stages 3 to 5 and 5D, randomized controlled trials have provided evidence that the use of sevelamer attenuates the progression of arterial calcifications compared with patients receiving calcium-based phosphate binders.429,439-441
Whereas neither cardiovascular nor all-cause mortality was reduced significantly by using sevelamer therapy in 1,068 patients completing the Dialysis Clinical Outcomes Revisited Study, the Renagel In New Dialysis Patients trial suggested a significant mortality reduction in incident dialysis patients receiving sevelamer for a median of 44 months.439,440
Lanthanum carbonate recently has become available as an alternative calcium- and aluminum-free binder with high affinity for phosphate and minimal intestinal absorption. In a randomized study in adult patients, lanthanum carbonate controlled plasma phosphate levels well and induced less adynamic bone disease than calcium carbonate.442 However, no long-term data about the effect of lanthanum on the functions of liver and kidney and bone, in which lanthanum accumulates,443 and its safety profile in children are available.
It should be emphasized that any phosphate-binder therapy introduces a major pill burden. The need to swallow several large tablets or capsules with each meal is a major physical and psychological challenge to many patients that can seriously compromise long-term adherence to this and other medications. Hence, phosphate-binder therapy should be individualized, realizing that in some patients, lowering of serum phosphate levels into the normal range may not be possible or may lead to an unacceptable decreased quality of life. In these cases, other options, such as intensified dialysis protocols, should be evaluated.