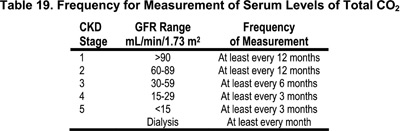
16.1 In CKD Stages 1-5, the serum level of total CO2 should be measured.
16.1.a The frequency of these measurements should be based on the stage of CKD as shown in Table 19. (OPINION)
16.2 In patients >2 years of age, serum levels of total CO2 should be maintained at ≥22 mEq/L (22 mmol/L); in neonates and young infants below age two, serum levels of total CO2 should be maintained at ≥20 mEq/L (20 mmol/L). (EVIDENCE) If necessary, supplemental alkali salts should be given to achieve this goal. Elevation of the bicarbonate concentration in the hemodialysis bath is an additional or alternative strategy. (OPINION)
Acidosis is a common component of many diseases of the kidney that affect the proximal or distal tubules, and is often present even when glomerular function is relatively intact (CKD Stages 1-2). Such diseases include inherited and genetic tubulopathies or acquired dysfunction of the tubules through, for example, the presence of obstructive uropathies or recurrent pyelonephritis. As glomerular function declines, acidosis may become more common and is uniformly present in CKD Stages 4-5. There is a developmental regulation of serum bicarbonate, such that values of ≥20 mEq/L (20 mmol/L) are normal for neonates and infants below two years of age, and values of 22 mEq/L (22 mmol/L) represent the lower limit of normal after age 2 years.
Chronic metabolic acidosis is a major component of the linear growth failure associated with CKD in infants and children with relatively preserved GFR. The mechanisms whereby acidosis blunts linear growth involve its effects on bone mineral on the growth hormone-IGF-I axis, and on renal synthesis of 1,25-(OH)2D, among others.
Classical studies in humans demonstrated the powerful effect of chronic metabolic acidosis, induced experimentally or resulting from CKD, on the loss of bone mineral. Experimental studies performed largely in animals fed excess mineral acid, or bone organ cultures exposed to varying pH environments, have investigated mechanisms whereby chronic metabolic acidosis alters bone composition. Chronic metabolic acidosis produces a change in the ionic composition of bone, with net reductions in apatite, sodium, and potassium. Cellular functions within bone are changed by chronic metabolic acidosis, such that matrix gene expression associated with osteoblastic activity is inhibited, while osteoclastic activities are increased. Additionally, the trophic effects of the growth hormone-IGF-I axis on bone growth and structure are blunted with chronic metabolic acidosis. Chronic metabolic acidosis reduces the kidney proximal tubule synthesis of 1,25(OH)2D, and may thereby limit the supply of calcium absorbed from the diet. Chronic metabolic acidosis alters the homeostatic relationships between blood ionized calcium, PTH, and 1,25(OH)2D such that bone dissolution is exaggerated.
Chronic metabolic acidosis contributes, in part, to the renal osteodystrophy in patients with CKD. Rickets is the most common manifestation of chronic metabolic acidosis in the bone of children with CKD Stages 1-3. The rickets may be cured by provision of alkali salts in some cases, but require supplemental vitamin D or its analogs in others. In adults, bone fractures are a relatively common manifestation of chronic metabolic acidosis. More recent studies in adults have demonstrated a reduction in bone mineral density, and in BFRs, by dynamic histomorphometry.540 Additional histomorphometric analyses of bone in patients with forms of chronic metabolic acidosis are quite limited, and remain controversial. Linear growth in children is reduced by chronic metabolic acidosis and successful treatment restores linear growth potential.541
There is scant evidence per se, in patients with kidney failure (adult or pediatric) and undergoing maintenance dialysis, that either amelioration or improvement of renal osteodystrophy occurs through elimination of chronic metabolic acidosis. However, a cross-sectional study of 76 adult patients studied with percutaneous, transiliac bone biopsy demonstrated that those with a normal biopsy result had a serum bicarbonate level ≥23 mM while those with either mild or advanced mixed osteodystrophy had serum bicarbonate levels <20 mM.542 It appears that the absence of acidosis renders therapy of renal osteodystrophy with a vitamin D metabolite more effective. Correction of metabolic acidosis allows normalization of linear growth in children with isolated renal tubular acidosis.541
Measurement and monitoring of the serum bicarbonate level is warranted in patients with proximal or distal tubulopathies or acquired tubulo-interstitial renal disease (such as from obstructive uropathies or recurrent pyleonephritis) regardless of glomerular filtration rates, in CKD Stages 1-5, and with maintenance dialysis. Measures to keep the bicarbonate level ≥22 mM (20 mM for the neonate and young infant below two years of age) are warranted for improvement in bone histology, and to improve linear growth. The clinician is reminded that the use of exogenous alkali salts containing citrate increases the absorption of aluminum in patients, and should be avoided as GFR declines into CKD Stage 3 and below (see Guideline 12).
Areas for future research into the effects of chronic metabolic acidosis and renal osteodystrophy include a fuller understanding of the calcium-vitamin D-PTH and the growth hormone-IGF-I axes in humans with CKD at the level of bone, especially at the growth plate. The role of newer therapeutic agents for treatment of osteoporosis in adults, such as bisphosphonates, selective estrogen-receptor modulators, or isoflavones in patients with CKD, with or without chronic metabolic acidosis, remains unknown.