CKD in patients with diabetes may or may not represent DKD. In the absence of an established diagnosis, the evaluation of patients with diabetes and kidney disease should include investigation into the underlying cause(s).
1.1 Patients with diabetes should be screened annually for DKD. Initial screening should commence:
- 5 years after the diagnosis of type 1 diabetes; (A) or
- From diagnosis of type 2 diabetes. (B)
1.1.1 Screening should include:
- Measurements of urinary ACR in a spot urine sample; (B)
- Measurement of serum creatinine and estimation of GFR. (B)
1.2 An elevated ACR should be confirmed in the absence of urinary tract infection with 2 additional first-void specimens collected during the next 3 to 6 months. (B)
- Microalbuminuria is defined as an ACR between 30-300 mg/g.
- Macroalbuminuria is defined as an ACR > 300 mg/g.
- 2 of 3 samples should fall within the microalbuminuric or macroalbuminuric range to confirm classification.
1.3 In most patients with diabetes, CKD should be attributable to diabetes if:
- Macroalbuminuria is present; (B) or
- Microalbuminuria is present
- in the presence of diabetic retinopathy, (B)
- in type 1 diabetes of at least 10 years' duration. (A)
1.4 Other cause(s) of CKD should be considered in the presence of any of the following circumstances: (B)
- Absence of diabetic retinopathy;
- Low or rapidly decreasing GFR;
- Rapidly increasing proteinuria or nephrotic syndrome;
- Refractory hypertension;
- Presence of active urinary sediment;
- Signs or symptoms of other systemic disease; or
- >30% reduction in GFR within 2-3 months after initiation of an ACE inhibitor or ARB.
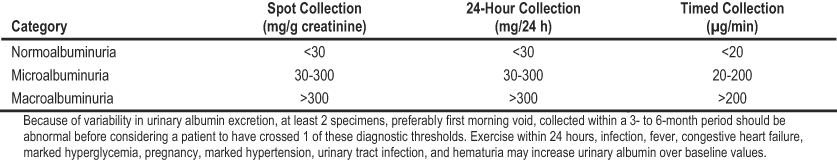
DKD, traditionally termed “diabetic nephropathy,” is a clinical diagnosis that historically has been based on the finding of proteinuria in a person with diabetes. This definition is independent of such markers of CKD as pathological change or a decreased GFR, and it initially was confined to those now considered to have macroalbuminuria. The development of more sensitive assays specific for albumin has since led to the detection of smaller increases, now termed microalbuminuria or “incipient nephropathy.” The lower limit of microalbuminuria is set somewhat arbitrarily at an albumin excretion rate (AER) of 20 µg/min, which is equivalent to 30 mg/24 h or an ACR of 30 mg/g (Table 3).222 These definitions have had some clinical utility in that individuals with macroalbuminuria historically had a progressive decrease in GFR associated with an increase in systemic blood pressure, whereas those with microalbuminuria were considered to have stable kidney function, yet were at high risk of subsequent development of macroalbuminuria and kidney failure.223
Table 3. Definitions of Abnormailities in Albumin Excretion

More recent information has led to a reevaluation of some of these concepts.224-226 The finding that a substantial proportion of patients with type 1 and type 2 diabetes and microalbuminuria spontaneously regress to normoalbuminuria calls into question the inevitability of kidney disease progression (Tables 4 and 5).224,226,227 The substantial variability in the severity of underlying pathology in type 1 diabetes228,229 and the heterogeneous nature of pathology in type 2 diabetes230 suggests that microalbuminuria may or may not reflect underlying DKD. Given the strongly positive relationship between the duration of diabetes and DKD, particularly in type 1 diabetes,231 the presence of elevated albuminuria in diabetes of short duration should raise concerns about non-DKD. Furthermore, although antihypertensive therapy reduces albuminuria, there is little evidence that it affects the underlying pathology, and short-term withdrawal of antihypertensive medicines can result in increases in albuminuria to pretreatment levels.208 Finally, the situation is complicated by the increasing use of microalbuminuria as a marker/predictor of CVD in people with and without diabetes. All these factors imply that the underlying mechanisms of albuminuria are multiple, not entirely pathology dependent, and do not fit neatly into definitions of CKD. Thus, any definition of DKD has to take all these factors into account.
Most professional societies concerned with diabetes and kidney disease now advocate screening for microalbuminuria in patients with diabetes.34,35 These recommendations have been made although there are no conclusive data that early intervention and treatment of microalbuminuria prevents CKD stage 5 or mortality in such patients.
Definitions
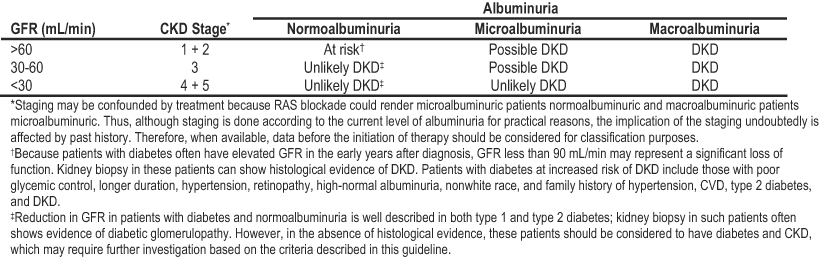
Definitions of DKD by albuminuria and stage are shown in Table 6. For this guideline, we included studies of people with type 1 or type 2 diabetes and CKD stages 1 to 5 regardless of whether kidney biopsies were performed. Studies of kidney transplant recipients were excluded. Because of the high prevalence of diabetes in the population, many individuals with other types of CKD also may have diabetes. Accordingly, the term DKD refers to a presumptive diagnosis of kidney disease caused by diabetes. The term diabetic glomerulopathy should be reserved for biopsy-proven kidney disease caused by diabetes.
Microalbuminuria and estimation of GFR satisfy criteria for a screening test for DKD. (Moderate)
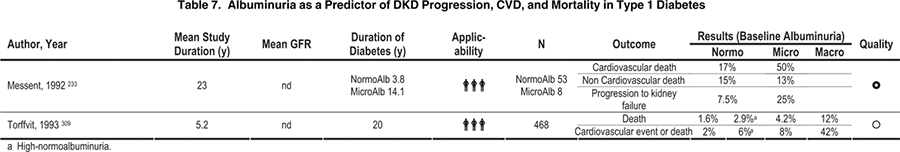
Microalbuminuria is an independent risk factor for the development of CKD 41, 232 and GFR loss223, 233 and for cardiovascular morbidity and mortality.234, 235 It is relatively common, and in studies using the cutoff points recommended in this guideline, the point prevalence of microalbuminuria varies (depending on the population) from 7% to 22% in type 1 236-238 and from 6.5% to 42% in type 2 134, 239-241 diabetes. Annual incidence rates of microalbuminuria of 1% to 2% are reported consistently for both type 1 and type 2 diabetes.
Tests for microalbuminuria are widely available, relatively inexpensive, and easy to perform. Because variations in urinary concentration caused by hydration status may adversely affect the interpretation of tests of albumin concentration alone and timed collections are inconvenient and prone to inaccuracy, the Work Group recommends estimating the ACR in a spot urine sample (preferably the first morning void).242
The sensitivity and specificity of ACR estimates are greater than 85% compared with timed urine collections.242 Some reported variation is dependent upon the method of albumin and creatinine measurement. Moreover, there is continuing debate around the effect of gender on the definition of normal values. Because women normally have lower urinary creatinine concentrations than men, their ACR values are higher for the same level of urinary albumin excretion. Accordingly, some investigators have recommended lower ACR cutoff values for normoalbuminuria in men than women. Whether sex-specific cutoff values improve accuracy is unknown and requires further study. Nevertheless, because urinary albumin excretion has an intraindividual CV of approximately 40%,36 multiple positive test results are required for classification.
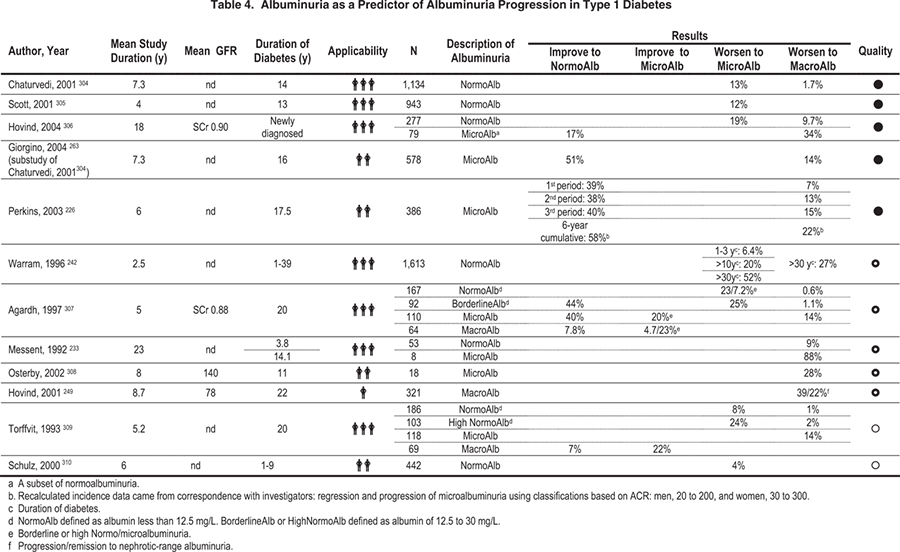
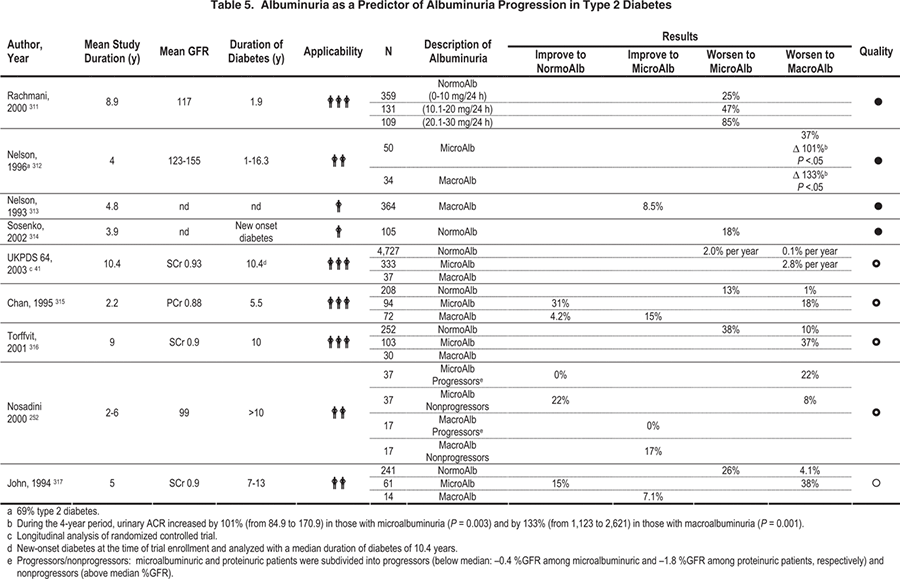
Table 6. Likelihood of DKD According to Staging by GFR and Level of Albuminuria
Although microalbuminuria satisfies nearly all criteria for a screening test, it does not satisfy the criterion of providing proven clinical benefits because the impact of microalbuminuria detection on such hard clinical end points as CKD stage 5, GFR loss, or CVD morbidity/mortality has not been demonstrated unequivocally (Table 7 and Table 8). Nevertheless, the ADA and other diabetes professional societies recommend annual screening for microalbuminuria based on the treatment possibilities discussed in CPR 1. The Work Group supports these screening recommendations while recognizing the need for further studies to define the impact of microalbuminuria detection on hard clinical end points. The suggested screening plan, adapted from the ADA guideline, is shown in Fig 6. 34,35
The evidence for the usefulness of eGFR alone as a screening test for CKD in diabetes is less secure. Many patients with diabetes and CKD may have elevated or high-normal GFRs, particularly in the early years after diagnosis. The same is true for all types of CKD. Whether values of GFR greater than 90 mL/min reflect progressive CKD may be determined best by the slope of sequential GFR estimates, rather than a single estimate. Therefore, markers of kidney damage are required to detect early stages of CKD; eGFR alone can detect only CKD stage 3 or worse. Although eGFR is recommended to classify patients with diabetes into stages of CKD (Table 6), some potential problems exist with the currently available estimating equations. The Modification of Diet in Renal Disease (MDRD) equation, presently the most widely used estimating equation for staging CKD, has been validated in only small numbers of patients with diabetes and CKD,243 and other equations may provide better estimates of GFR in these patients.244 An NIH-sponsored study currently is ongoing with the purpose of developing a new equation derived from multiple databases along with extensive calibration studies to ensure generalizability throughout the entire range of GFRs.
Despite their value in the vast majority of patients, currently recommended screening tests are not sufficient to identify all cases of DKD because serious diabetic glomerular lesions may occur in normoalbuminuric patients with normal GFR.228 Normoalbuminuric patients with decreased GFR have even more severe glomerular changes.245,246 Therefore, further evaluation, including consideration of kidney biopsy, may be required in some cases to establish the diagnosis of DKD.
Screening for kidney disease should begin 5 years after the diagnosis of type 1 diabetes and at the diagnosis of type 2 diabetes. (Moderate/Strong)
Although transient increases in albuminuria in newly diagnosed type 1 diabetes are well described, it is thought that this increase represents acute metabolic perturbations and the level of albuminuria usually reverts to normal after glycemic correction. Most longitudinal cohorts report significant increases in microalbuminuria prevalence only after 5 years' duration, although 1 cross-sectional study described a significant prevalence of around 15% in patients with 1 to 5 years of diabetes.236 Conversely, the UKPDS found a urinary albumin concentration greater than 50 mg/L in 6.5% of newly diagnosed, mainly white patients with type 2 diabetes.134 This group suggested an average 8-year delay in diagnosis of type 2 diabetes from the onset of beta cell failure and hyperglycemia. Moreover, 28% of these patients had hypertension at diagnosis. Accordingly, whereas screening can wait until 5 years after the onset of type 1 diabetes, the inability to establish the onset of type 2 diabetes with certainty makes screening at diagnosis mandatory.
Elevated ACR should be confirmed in the absence of urinary tract infection. (Moderate)
AER has a high day-to-day variability, probably reflecting the multiple factors that can influence the appearance of albumin in the urine.36 These include such metabolic perturbations as ketosis and hyperglycemia and such hemodynamic factors as physical exercise, dietary protein intake, diuresis, and the presence of urinary tract infection. Because of this variability, most professional societies recommend confirmation of an elevated ACR with an additional 2 tests during the subsequent 3 to 6 months (Fig 6).34,35 To reduce variability, these repeated estimates should be performed on first-voided urinary specimens.
In most people with diabetes, CKD should be attributable to DKD in the presence of: (1) macroalbuminuria or microalbuminuria plus retinopathy, and (2) in people with type 1 diabetes, in the presence of microalbuminuria plus duration of diabetes longer than 10 years. (Moderate/Strong)
Historically, detection of macroalbuminuria was the basis of the diagnosis of DKD (Table 6). Kidney biopsy in macroalbuminuric patients with type 1 diabetes consistently shows advanced diabetic lesions of increased mesangial volume, increased glomerular basement membrane thickness, and tubulointerstitial pathologies.228,229, 247,248 The severity of these abnormalities is related closely to the amount of albuminuria and the decrease in GFR (Table 9 and Table 10). GFR decreases relentlessly at rates greater than 10 mL/min/y in those with poorly controlled hypertension and macroalbuminuria, but much more slowly (1 to 4 mL/min/y) in those with effective blood pressure control.249,250
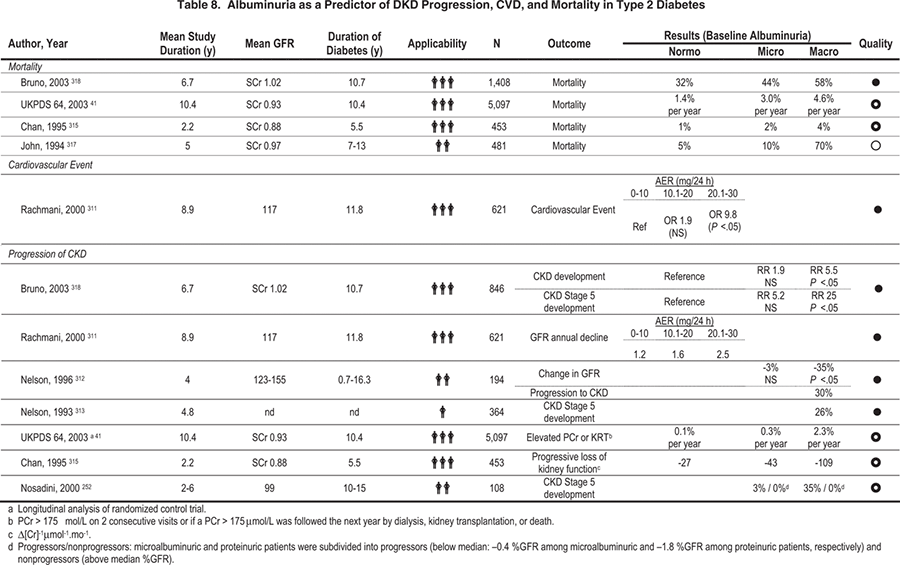
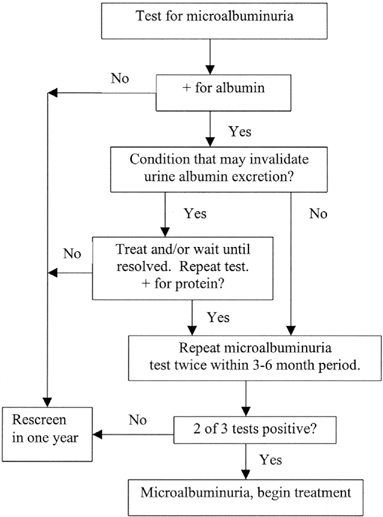
Figure 6. Screening for microalbuminuria.
Reprinted with permission.35
In microalbuminuric patients with type 1 diabetes, pathological lesions tend to be less severe than in macroalbuminuric patients, but usually are significantly more advanced than those seen in normoalbuminuric individuals, particularly in the presence of hypertension.228, 229 GFR is stable at low-level microalbuminuria, but decreases at 1 to 4 mL/min/y as AER increases, and more rapidly in those with poorly controlled hypertension.223
The situation in type 2 diabetes is less clearcut, with only about 40% of microalbuminuric patients who undergo biopsy for research purposes showing diabetic changes typical of those seen in patients with type 1 diabetes.230 About 30% of them have normal or near-normal biopsy results, whereas the other 30% have increased severity of tubulointerstitial, vascular, and/or glomerulosclerotic lesions unrelated to classic diabetic glomerulopathy.230 In general, the kidney structural-functional relationships established in type 1 diabetes hold in type 2 diabetes (Table 11 and Table 12), but the correlations are less precise, especially because of a sizeable cluster of patients with type 2 diabetes and microalbuminuria or proteinuria with little or no diabetic glomerulopathy lesions.230, 251 The rate of GFR decrease in patients with type 2 diabetes, microalbuminuria, and proteinuria is greatest in those with typical diabetic glomerular lesions.252
The concomitant presence of retinopathy is only partly helpful in discriminating kidney pathology in patients with type 2 diabetes (Fig 7 ; Table 13).147, 251, 253-262 In those with macroalbuminuria, the positive predictive value (PPV) of retinopathy for typical diabetic glomerulopathy ranges from 67% to 100%. However, the negative predictive value (NPV) had a broader range of 20% to 84%. These figures give sensitivities between 26% and 85% and specificities of 13% to 100%. For microalbuminuria, PPVs were lower at around 45%, but NPVs were close to 100%, giving sensitivities of 100% and specificities of 46% to 62%. Thus, the presence of retinopathy in patients with type 2 diabetes and macroalbuminuria is strongly suggestive of DKD, and its absence in microalbuminuria suggests non-DKDs. Only a small number of patients in these series were found to have non-DKD amenable to a specific therapy, and most of those individuals had other clinical features, such as nephrotic syndrome or nondiabetic systemic illness.
Duration of diabetes is related closely to the prevalence of DKD in type 1 patients. Prevalence rates of microalbuminuria and macroalbuminuria increase after 10 years, presumably reflecting cumulative glycemic exposure (see Guideline 2). Patients with type 1 diabetes, microalbuminuria, shorter diabetes duration, lower AER levels, better glycemic control, and lower blood pressure and plasma lipid concentrations are more likely to reverse to normoalbuminuria.226, 263, 264 The contribution of the prepubertal years of diabetes to DKD risk may be lower than that of postpubertal years, but this remains controversial.265-270 However, there are few good data on comparative levels of glycemic control in young children, making it difficult to control for this variable. There also may be a nonlinearity of risk of pathological damage before achievement of full growth, but this risk may be duration dependent, rather than puberty dependent.271 Moreover, postural proteinuria may be more common during adolescence, making the diagnosis of DKD more uncertain and the recommendation for screening by using overnight urine collections especially important in this age group. For these and other reasons, it would be incorrect in the view of the Work Group to regard the prepubertal period as risk free for the development of DKD. This topic needs additional research. Because of the clinical difficulty accurately determining the onset of type 2 diabetes, known duration is less strongly related to DKD. In Pima Indians, the duration of type 2 diabetes is known with greater accuracy and precision because of systematic screening for diabetes, and in this population, the duration of diabetes is as strongly related to DKD as in type 1 diabetes.272
Several small series of patients with type 1 and type 2 diabetes describe cases of typical diabetic glomerulopathy with normoalbuminuria and normal or decreased GFR. These data bring into question the reliance on increased AER alone or in combination with GFR for diagnosis of DKD. Most of these patients were women, had relatively long durations of diabetes, and usually had retinopathy and/or hypertension.245, 246, 273
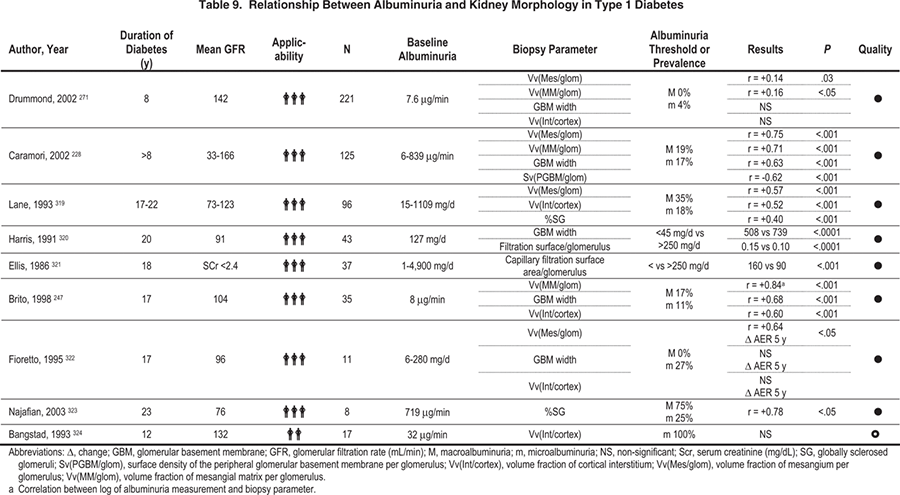
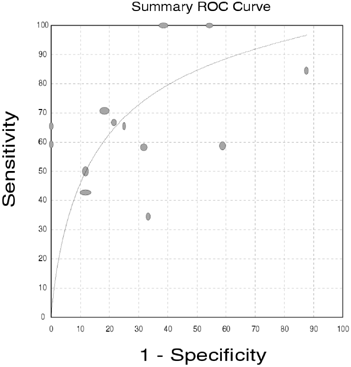
Figure 7. Receiver operator characteristic (ROC) curve of the probability that the presence of diabetic retinopathy is predictive of patients who have biopsy/histology-proven diabetic glomerulopathy. Each ellipse represents an individual study, for which the height and width of the ellipse is representative of the inverse variance of the sensitivity and specificity, respectively.147, 251, 253-262
Atypical clinical features should prompt evaluation for non-DKD. People with diabetes and CKD may have increased risks of testing and treatments. (Moderate)
Because diabetes is a common condition, coincidence with other nondiabetic CKD is relatively frequent. Accordingly, evaluation of a person with atypical features should include additional diagnostic testing in selected cases, depending on the clinical presentation. For example, because generalized vascular disease is common in diabetes, refractory hypertension and/or a significant decrease in kidney function after RAS blockade should prompt consideration of renal artery stenosis. Rapidly decreasing kidney function and/or increasing proteinuria (particularly if nephrotic), active urinary sediment, or evidence of other systemic disease should raise concerns about nondiabetic glomerular disease. Diagnosis of these diseases may require invasive testing or interventional procedures. Care should be used in determining the appropriate diagnostic tests because administration of radiographic contrast, with or without angiography, may pose greater risks in people with diabetes and CKD than in other people.
It is the opinion of the Work Group that in the absence of another identifiable and treatable cause of kidney disease, patients with diabetes and CKD should be treated as if they have DKD.
A kidney biopsy may be required in some patients with diabetes and CKD to determine the underlying cause of the kidney disease. (Moderate)
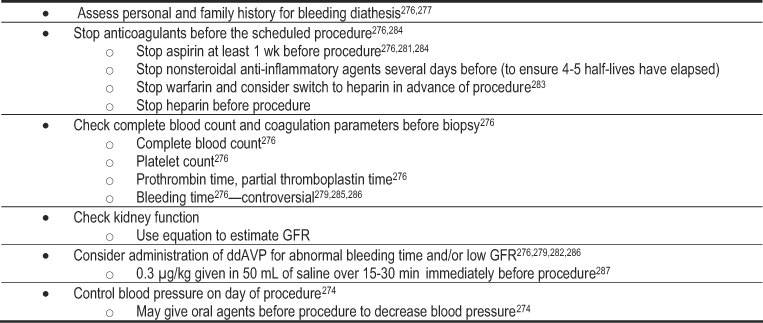
The risk of complications associated with percutaneous native kidney biopsy in patients with DKD is no greater than the risk faced by patients with most other causes of CKD.274, 275 The majority of complications are from bleeding and include microscopic hematuria, decrease in hemoglobin level, gross hematuria, perinephric hematomas, and arteriovenous fistulae.276, 277 Women are more likely to bleed than men, and other commonly identified risk factors for bleeding include younger age, decreased GFR, elevated systolic and diastolic blood pressure, and prolonged bleeding and partial thromboplastin times.274, 276, 278, 279 The number of needle passes during kidney biopsy also increases the risk of bleeding, particularly if the number exceeds 4 276 or 5 passes.274 Use of real-time imaging appears to improve the success and safety of the procedure.276, 280 To reduce the risk of bleeding complications,274, 276, 278, 279, 281-287 anticoagulant medicines should be stopped in advance of the biopsy, blood pressure should be controlled, and 1-deamino-8-D-arginine (ddAVP) may be given immediately before the procedure if bleeding time is prolonged (Table 14).
Caution should be used when administering radiographic contrast agents to patients with diabetes and CKD because their risk of RCN is higher than in those without these diseases. (Moderate/Strong)
RCN is identified by both a change in kidney function (eg, GFR or serum creatinine level) and the time course over which kidney function changes. A standard definition of RCN does not exist, but definitions used in previous studies have included increases in serum creatinine concentration ranging from 0.5 mg/dL to a doubling of the concentration and decreases in GFR ranging from 25% to dialysis requirement.288-290 In general, most studies assess kidney function within 48 to 72 hours after contrast administration. Serum creatinine concentration usually increases within 48 hours of radiographic contrast administration and peaks within 7 days.
The lack of a standardized definition of RCN makes comparisons between studies difficult. Nevertheless, the risk of RCN is higher in people with diabetes and CKD than in either condition alone. In general, the incidence of RCN is less than 3% in patients with neither diabetes nor CKD, 5% to 10% in those with diabetes, 10% to 20% in those with CKD (greater at later stages), and 20% to 50% in those with both diabetes and CKD (Table 15).291, 292
Patients who develop RCN have greater mortality, both short and long term, than those who do not.293,294 Accordingly, efforts to prevent or minimize RCN should be implemented in those with diabetes and CKD. However, the evidence for prevention of RCN in these patients is relatively limited (Table 16 and Table 17). Many studies do not report incidence of RCN by the presence of diabetes and CKD, and those that do often are derived from subgroup analyses so the number of patients is small. Despite these limitations, several strategies have been developed that may reduce RCN risk in people with diabetes and CKD, as well as in other populations. First, concomitant nephrotoxins (eg, nonsteroidal anti-inflammatory agents, aminoglycosides, and amphotericin) should be discontinued, if possible, before administering the radiographic contrast agent.295 Second, intravenous fluids should be administered, but caution should be used in determining the amount of fluid to avoid fluid overload. Most studies evaluated 0.45% sodium chloride at a dose of 1 mL/kg/h over 6 to 12 hours, but they did not include patients with advanced CKD.296 A recent study suggested that 0.9% sodium chloride may be better than 0.45% sodium chloride for preventing RCN.298 Third, a greater volume of contrast is associated with an increased risk of RCN. In the general population, administration of more than 100 mL of hyperosmolar radiographic contrast increases the risk of RCN, but in those with diabetes and an eGFRless than 30 mL/min/1.73 m2, as little as 30 mL of radiographic contrast agent can lead to acute kidney failure.298 Hence, the use of radiographic contrast material should be kept to the minimum amount necessary for the evaluation required.297 Fourth, the type of contrast material affects the risk of RCN. Nonionic radiographic contrast material may confer a lower risk of RCN than ionic contrast material.287 Moreover, a randomized controlled trial reported that iso-osmolar radiographic contrast (eg, iodixanol) is associated with significantly lower incidences of RCN than a low-osmolar contrast agent in patients with diabetes and CKD.288 Fifth, because lactic acidosis may occur with RCN in patients with diabetes receiving metformin, this medicine should be withheld for 48 hours before infusion of contrast medium and after exposure, until the estimate or measure of GFR is greater than 40 mL/min/1.73 m2.299 Use of metformin is not recommended in patients with diabetes and CKD (see Guideline 2).
Table 14. Strategies to Prevent Bleeding After Kidney Biopsy
Although there is much interest in finding medicines to prevent RCN, few are known to be beneficial and none has been studied in a large population of patients with diabetes and CKD. Table 18 summarizes the clinical trials that report results in patients with diabetes and CKD. Studies examining the effectiveness of N-acetylcysteine, sodium bicarbonate, and hemofiltration have not specifically reported results for patients with diabetes and CKD. Nevertheless, in the opinion of the Work Group, it is reasonable to consider these approaches for people with diabetes and CKD, considering their high risk of RCN (Table 18).295, 300-302
The European Society of Urogenital Radiology299 and the American College of Radiology (www.acr.org; last accessed January 31, 2006) offer guidelines for use of contrast media. These guidelines and results of a number of clinical trials are described in a recent review of methods for preventing RCN. The American guidelines mention the use of N-acetylcysteine and other potential prophylactic drug therapies without specifically recommending these approaches.
Table 15. Observed Incidence of Acute Kidney Failure After PCI That Included Administration of Radiocontrast, Stratified by Baseline Serum Creatinine and Diabetes Status292
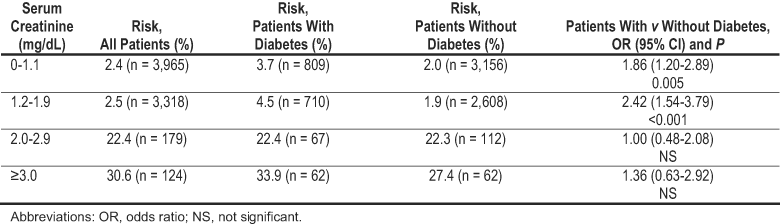
Table 18. Preventive Strategies for RCN

No data are available to confirm that detection of microalbuminuria and initiation of treatment at this early stage of DKD leads to a decrease in hard end points (GFR decrease, CKD stage 5, and mortality). Furthermore, the predictive value of microalbuminuria for DKD is not as high as originally considered. Whether the lower predictive value is due to changes in disease natural history, improved therapies, or overestimation by the original studies is uncertain.224 However, as many as 30% to 50% of microalbuminuric patients may revert to normoalbuminuria,224, 226, 263, 264 and whether this regression is spontaneous or not cannot be determined if the patient is on ACE inhibitor or ARB treatment. Nevertheless, some data suggest benefit of intensive glycemic and blood pressure control in patients with microalbuminuria. A detailed discussion of treatment of albuminuria (microalbuminuria and macroalbuminuria) and evaluation of outcomes can be found in CPR 1.
The current recommendations for microalbuminuria screening by the ADA34,35 do not specifically recommend use of a first morning urine sample or overnight collections. However, postural microalbuminuria or proteinuria may be a confounding factor, particularly in young type 1 patients. Despite these limitations, it is clear that patients who are persistently normoalbuminuric tend to be at low risk of DKD, whereas microalbuminuric patients have a 3- to 4-fold increased risk. For classification purposes, the Work Group recommends that health care providers consider as macroalbuminuric all patients who have been diagnosed as such before ACE-inhibitor and/or ARB treatment.
Another limitation of this guideline relates to the classic definition of DKD according to AER, which has been used in the vast majority of treatment trials (see CPR 1). AER does not map easily to the KDOQI™ stages of CKD (Table 6) because staging is based on eGFR. Thus, while GFR may be elevated or within the normal range in people with elevated urinary albumin excretion, loss of GFR within CKD stage 1 may already represent DKD. The formulae estimating GFR from serum creatinine values are problematic in their application to patients with diabetes.244 Nonetheless, measures of albuminuria in combination with estimates of GFR will serve as useful guides in assessing and managing patients with diabetes and CKD. The Work Group developed a novel grid (Table 6) to combine staging by albuminuria classification and GFR, although at this time, evidence to define DKD probabilities within each box of this table is lacking.
The diagnosis and staging of DKD in an individual patient should include an evaluation of other related factors. Apart from albuminuria and GFR, patients should be evaluated for the presence of hypertension, poor glycemic control, dyslipidemia, and smoking. A family history of DKD or hypertension and/or CVD and stroke in parents without diabetes also is relevant. Moreover, in patients developing DKD, hypertension and dyslipidemia may be risk predictors, concomitants, or consequences. Because DKD typically does not occur in isolation, patients with DKD should have regular surveillance for other microvascular and macrovascular complications. These issues are covered in more detail elsewhere in these guidelines under the sections relating to background, blood pressure control, glycemic control, lipid management, lifestyle issues, and multifactorial intervention.
Ideally, ACR should be measured in first-void urine samples, but sometimes this may be impractical. Alternatively, if a random urine specimen is abnormal, the second test could be done in a first-voided morning sample obtained within the subsequent 3 to 6 months. Screening for microalbuminuria in patients with type 2 diabetes, if leading to multifactorial interventions, can result in reduced risks of cardiovascular events, progression of albuminuria, and development or progression of retinopathy and neuropathy.303 Similar studies in patients with type 1 diabetes are lacking. Several cost-benefit analyses of screening for microalbuminuria have been published using various models. These models refer mostly to type 1 diabetes and have not been confirmed prospectively in clinical trials. International standards for measurement of creatinine and albumin should be adopted, and quality control between laboratories should be established. There should also be standardized reporting of ACRs with internationally agreed-upon categorical definitions.