12.1 In all patients with baseline serum aluminum levels >60 µg/L, a positive DFO test, or clinical symptoms consistent with aluminum toxicity (Guideline 11, Table 31), the source of aluminum should be identified and eliminated. (OPINION)
12.2 In symptomatic patients with serum aluminum levels >60 µg/L but <200 µg/L or a rise of aluminum after DFO >50 µg/L, DFO should be given to treat the aluminum overload. (See Algorithm 8 and Algorithm 9.) (OPINION)
12.3 To avoid DFO-induced neurotoxicity in patients with serum aluminum >200 µg/L, DFO should not be given until intensive dialysis (6 days per week) with high-flux dialysis membrane and a dialysate aluminum level of <5 µg/L and until the pre-dialysis serum aluminum level has been reduced to <200 µg/L. (OPINION)
When dialysis encephalopathy and dialysis-related bone disease were first recognized, most patients had progressive disease with profound morbidity and very high mortality. The early cases arose, in large part, due to aluminum-contaminated dialysate. However, most patients were also receiving aluminum gels to control hyperphosphatemia, as it was then believed that little or none of the aluminum was absorbed. The first successful reversal of symptoms of dialysis encephalopathy were observed with DFO given in doses of 20 to 40 mg/kg for treating patients with aluminum-related bone disease. There was clinical and histological improvement; however, immediate side-effects affecting vision and mental status appeared in isolated patients, and there was concern about the use of DFO. More ominous was the appearance of rapidly progressive and fatal mucormycosis in dialysis patients who had been receiving DFO treatment. At about the same time, there was the introduction of calcium-based phosphate binders as well as widespread purification of water used for dialysate, so the prevalence of severe aluminum toxicity seemed to diminish. However, some aluminum toxicity still occurs and there remains a question of when and how chelation therapy with DFO should be used.
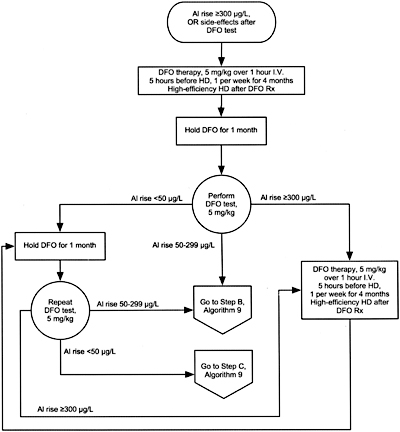
Algorithm 8. DFO treatment after PAl rise =300 µg/L.
Algorithm 9. DFO treatment after PAl rise between 50 and 300 µg/L.
Beneficial Effect of DFO Treatment on Aluminum Bone Disease and Other Features of Aluminum Toxicity
Long-term DFO treatment reduces the surface-stainable aluminum on trabecular bone.430,450-453 This is associated with an increase of bone formation rate,430,450-452 and symptoms of proximal muscle weakness and bone pain commonly improve.450,454,455 Isolated reports have shown improved neurological symptoms in patients with dialysis encephalopathy.456-460 In these reports, DFO doses have varied from 1 to 6 g430,454 or, expressed in relation to body weight, 30 to 40 mg/kg BW per treatment.451,452,461 The treatment was given once weekly in some trials,430,455 and with each dialysis (thrice weekly) in others.451,452,461 In 1 study,430 the reduction of stainable aluminum and improved bone formation rates were substantially less in patients with an earlier parathyroidectomy than in those with intact parathyroid glands. Treatment with DFO was associated with improvement of anemia in some, but not all, patients.453,461,462
Side-Effects of DFO Treatment
Two serious problems associated with DFO therapy are: (1) the precipitation of acute aluminum neurotoxicity and (2) the development of mucormycosis, which is commonly fatal.
Precipitation of acute aluminum neurotoxicity.
When DFO is given to patients with very high serum aluminum levels (>200 µg/L), acute and fatal aluminum neurotoxicity has been precipitated411,412; this presumably occurs because aluminum is rapidly mobilized from various tissue stores.
Fatal mucormycosis in dialysis patients receiving DFO.
In experimental infections with Mucor species, DFO administration markedly augments the growth and pathogenicity of the mucormycosis.463,464 When DFO is given, it chelates iron to form feroxamine; the latter has a molecular weight of 714 Da and several dialysis treatments are needed to clear it from the circulation. Certain species of Mucor, with very low pathogenicity, exist widely in nature and are found on skin and mucous membranes; feroxamine enhances their growth and pathogenicity, thereby promoting the development of fatal disseminated or rhinocerebral mucormycosis in hemodialysis patients given DFO.320,435,465 Most afflicted patients had received DFO, 20 to 40 mg/kg BW, once or thrice weekly, with standard dialysis membranes (usually cuprophane) employed. The shortest reported duration of treatment before infection appeared was 3 weeks.435
Methods to avoid serious side-effects. In patients exposed to high dialysate aluminum levels or with high plasma aluminum levels (>120 µg/L), the following scheme is recommended to reduce the risk of acute neurotoxicity:
The very first dose of DFO is withheld until serum aluminum levels are substantially reduced after total withdrawal of aluminum exposure—both from dialysate and from ingesting aluminum-containing drugs. With serum aluminum levels >200 µg/L, daily hemodialysis should be done using high-flux membranes and dialysate aluminum concentration <5 µg/L. The first “low dose” DFO test (5 mg/kg) should be done only after 4 to 6 weeks of such treatment, with increment of plasma aluminum determining the timing of subsequent DFO treatments. If the increment of aluminum is high (>300 µg/L), DFO treatments should be given via a peripheral vein, 5 hours before the next dialysis that uses a high-flux membrane; this allows for rapid removal of the DFO-aluminum complexes from the circulation and minimizes the duration of patient exposure to high concentrations of the DFO-aluminum chelate (aluminoxamine).
Fig 14. Individual study and summary effect sizes for the effect of DFO therapy on bone formation rate.
If the increment of plasma aluminum after the first DFO test is <300 µg/L and no neurological or ophthalmological symptoms appear, the DFO can be given over the last hour of dialysis, with the next dialysis done using a high-flux dialyzer, 44 hours later. The dose of DFO should be 5 mg/kg, with an expanded interval between treatments of 3 to 4 dialysis procedures using a high-flux hemodialysis membrane; this allows for more complete clearance of feroxamine from the circulation, reducing the risk of mucormycosis. Intravenous iron should be avoided while DFO is being given to limit the formation of feroxamine.435,463
Management of aluminum overload without symptoms.
The proper management of aluminum overload in the absence of symptoms is not established. There have been "consensus" viewpoints that aluminum overload be treated with DFO466; however, there are no data to support this recommendation. When CKD Stage 5 patients with aluminum overload and high plasma aluminum levels have aluminum gels withdrawn and they undergo dialysis with aluminum-free dialysate (<5 µg/L), plasma aluminum levels fall substantially and progressively.203,442,444 Small numbers of patients with histomorphometric features of aluminum bone disease but without any musculoskeletal symptoms were treated as above; after 1 year, repeat bone biopsies showed a reduction of surface stainable aluminum and a rise in bone formation rate consistent with reversal of aluminum bone disease.467,468 The exception was 2 patients who had previously undergone parathyroidectomy; in these 2 patients, there was a modest reduction of surface-stainable aluminum but bone formation rate did not improve to normal.468 These data suggest that DFO therapy may not be needed for the treatment of asymptomatic patients.
Beneficial Effects of DFO Therapy. Several trials with DFO therapy showed a reduction of surface aluminum staining,430,451-454 and an increase in bone formation rate,430,451,452,454 after treatment periods of 8 to 12 months. Meta-analysis of 4 trials that provided data on aluminum staining and 3 trials with bone formation rate are shown in Figs 14 and 15 , respectively. The doses used were variable, ranging from 20 to 40 mg/kg; there are no data to indicate a benefit of thrice-weekly treatment compared to once weekly. All these trials utilized standard dialysis membranes (probably cuprophane). The data on improvement of neurological features of dialysis encephalopathy involve many reports of small numbers of patients who received such treatment.456-460,469-471
Fig 15. Individual study and summary effect sizes for the effect of DFO therapy on bone surface aluminum stain.
Data on the most efficient means to clear DFO-bound aluminum from the circulation include dialysis using a high-flux membrane472 or hemoperfusion with a charcoal filter473; these remove aluminum more rapidly than standard dialysis using cuprophane membranes. A crossover study compared: (1) the combination of charcoal perfusion combined with standard dialysis; (2) dialysis using a high-flux membrane; and (3) standard dialysis. The hemoperfusion/hemodialysis combination had a small advantage over the high-flux dialyzer,474 and standard dialysis was inferior to both. In this study, the removal of feroxamine (the DFO-iron complex) was far greater with either the high-flux dialyzer or the hemoperfusion/hemodialysis combination than with the standard cuprophane dialyzer. Other studies showed that either intraperitoneal or intramuscular administration of DFO was effective in augmenting aluminum removal in patients undergoing peritoneal dialysis.475,476 The intramuscular administration of DFO, as it is sometimes given in hematological disorders, may provide a convenient method 4 to 5 hours before dialysis when an intravenous route is not available.476
Experience with the "safe" long-term treatment with DFO is derived from an outbreak of marked aluminum loading due to aluminum contamination of water used to prepare the dialysate solution. A 6-month course of "low-dose" DFO treatment was used in 42 patients exposed to high dialysate aluminum.477 After neurological symptoms first appeared, but before the diagnosis of aluminum intoxication was made, 11 patients had died. Forty-two other patients were followed. All aluminum gels were stopped, a new reverse-osmosis system was installed, and an alternate water source was used (dialysate Al <2 µg/L). The initial basal aluminum levels were 506 ± 253 µg/L (mean ± SD; range, 104 to 1,257 µg/L); hemodialysis was done for 4 hours, 6 days per week; charcoal hemoperfusion was combined with the dialysis weekly. (High-flux dialysis membranes, which had similar clearance of DFO-stimulated aluminum as hemoperfusion, were not available.) After 4 weeks, the frequency of dialysis was reduced to thrice weekly with hemoperfusion once weekly.
After 6 weeks of such “intensive hemodialysis/hemoperfusion,” the basal serum aluminum fell from 506 ± 253 µg/L to 121 ± 46 µg/L (mean ± SD). The first DFO infusion test (5 mg/kg) was given during the last hour of dialysis; the increment of plasma aluminum was 300 µg/L in 11 patients, 7 of whom developed neurological symptoms (headache, hallucinations, or myoclonic jerks) and 2 developed ophthalmological symptoms (transiently blurred vision) after the DFO test; 30 patients had increments of plasma aluminum <300 µg/L, only 3 of whom had developed neurological symptoms. These 3 symptomatic patients and the 11 patients with a post DFO aluminum increment <300 µg/L received DFO treatment given via a peripheral vein 5 hours before starting a hemodialysis/hemoperfusion session (Group 1). The other 27 patients (Group 2) received DFO (5 mg/kg) during the last hour of dialysis with a hemodialysis/hemoperfusion session done 44 hours later. The DFO treatments were given weekly in all patients. After 4 months, DFO was stopped for a 4-week “washout,” and the DFO test was repeated. If the basal plasma aluminum was <60 µg/L and the increment after DFO was <50 µg/L, the DFO treatment was stopped (2/14 of Group I and 8/27 of Group 2). If the basal serum aluminum level or the increment exceeded these limits, DFO treatment was continued weekly for an additional 2 months. There have been no comparisons of different doses of DFO, although cross-over studies with single infusions and small short-term studies suggest that doses lower than 5 mg/kg may be useful.
Throughout this 6 months of DFO treatment, no neurological or ophthalmological symptoms appeared and the baseline plasma aluminum gradually fell, as did the increment after DFO. There were significant increments in the mean cell volume (MCV) of RBCs and a modest rise in plasma intact PTH levels in both groups.478
Mucormycosis and DFO Treatment. Numerous case reports have described fulminant, fatal cases of systemic or rhinocerebral mucormycosis in dialysis patients being treated with DFO for aluminum toxicity,320,435,465,479,480 while reports of mucormycosis among dialysis patients not receiving DFO are unusual.391 An international registry collected 59 cases of mucormycosis among dialysis patients435; among these, 78% had been treated with DFO for aluminum or iron overload. In this report, the mortality was 91%, the disorder was the disseminated or rhinocerebral variety in 75% of the cases, and a diagnosis of mucormycosis was made only at autopsy in 61%. Experimental infections with mucormycosis in animals demonstrated that DFO, and in particular feroxamine, augmented the pathogenicity of certain species of Mucor and prevented effective treatment with amphotericin B.463,464,481 Increased susceptibility to mucormycosis was found to occur because of persistence of significant concentrations of feroxamine, the iron chelate with DFO, in ESRD patients given DFO. Such feroxamine is rapidly excreted by the kidneys of hematology patients treated with the drug, and mucormycosis has been very rare among DFO-treated hematology patients with normal kidney function.391 The clearance of feroxamine by a standard dialyzer is quite low, and 3 to 4 dialysis treatments may be required to clear this substance from the blood.474
With proper water purification and reduction in the intake of aluminum gels, the incidence of aluminum bone disease and other features of aluminum toxicity has decreased substantially. Over the same period, there have been trials utilizing much lower doses of DFO to treat aluminum toxicity. Also, there appeared to be fewer cases of mucormycosis in 1986 through 1989 as the DFO usage decreased. For a recent review of mucormycosis, an attempt was made to locate cases that had occurred over the last 10 years; in communications with various individuals in Belgium, Spain, Portugal, and the United States with interest in aluminum toxicity and use of DFO, no recent cases of mucormycosis associated with DFO therapy could be identified.482
Attention has been given to methods that utilize DFO in a manner that reduces its risk. By reducing the time between the administration of DFO and the next dialysis, and by doing the dialysis with a highly permeable membrane, both feroxamine and the aluminum chelate, aluminoxamine, are removed more effectively.472 Hemoperfusion with a sorbent cartridge has also been very effective474; however, such cartridges are not presently available in the United States. In addition, there has been a reduction of the DFO dose from 20 to 40 mg/kg to 5 to 10 mg/kg, with the DFO dose given 4 to 6 hours before the next dialysis, along with the use of a high-flux or highly permeable dialysis membrane and/or the use of a sorbent system.477 Also, DFO should only be given every 7 to 10 days,477 with 3 to 4 dialysis procedures between each dose of DFO.477 With attention to prevention of aluminum toxicity by curtailing the administration of aluminum-containing drugs and attention to proper water purification, the incidence of aluminum toxicity is now much lower than it was 10 to 15 years ago. It has not been possible to identify patients who have developed mucormycosis when these newer protocols have been followed.
The trials showing beneficial effects of DFO treatment on bone biopsies and symptoms of aluminum bone disease were done several years ago when symptomatic aluminum bone disease was common, and most used DFO in doses of 20 to 40 mg/kg BW. Despite this, the numbers of patients in prospective trials was relatively small; also, because of the severity of the disorder and poor prognosis in untreated patients, there were no controlled trials. In a small trial of asymptomatic patients found to have biopsy evidence of aluminum bone disease, there was reduced surface staining of aluminum and increased bone formation when all exposure to aluminum was eliminated.468 There is evidence in one trial that the use of DFO in a dose of 5 mg/kg is effective in lowering basal serum aluminum. The largest trial represented acute marked aluminum loading, and neurological rather than skeletal disease was the major risk. Fourteen patients who were not treated died, and there was only 1 death (due to hyperkalemia) among 42 patients who followed the recommended protocol.405 Small studies suggest that doses of DFO as low as 1 mg/kg and 0.5 mg/kg can raise the ultrafilterable aluminum in plasma, so such aluminum can be removed by dialysis, but there are no long-term data documenting the effectiveness of such low doses.
With regard to the safety of using low doses of DFO, the reduced frequency of its administration to once weekly, and use of high-flux dialyzers to minimize the increased susceptibility to mucormycosis, the evidence is only indirect. It has not been possible to find any cases of mucormycosis with this schema. If no new cases of fatal mucormycosis appear, this will be presumptive evidence of the effectiveness of the preventive measures.
The management of dialysis patients with "asymptomatic aluminum loading" has not been carefully evaluated, and the recommendation of treating such patients with DFO466 has not been critically evaluated. There was a small number of dialysis patients with elevated plasma aluminum levels and histological features of aluminum bone disease, who had repeat biopsies approximately 12 months after all aluminum was withdrawn.467,468 The close association between the reduction of surface stainable aluminum and the improvement of bone formation and mineralization rate468 is consistent with a "cause and effect" relationship. Whether such patients would have had greater improvement after receiving DFO treatment is uncertain. The lack of improvement of bone formation in the patients with earlier parathyroidectomy468 is consistent with failure of bone histomorphometry to improve in DFO-treated patients with symptomatic aluminum bone disease.430
The proper and early identification and treatment of aluminum toxicity, even that occurring accidentally via unusual contamination of dialysate or water, is now possible and safe using the low doses of DFO that are recommended. Although prevention of aluminum toxicity is greatly preferred, the early recognition and initiation of aggressive treatment might reduce the very high mortality associated with acute aluminum neurotoxicity when patients are seen in the early phases and treated with daily, high-efficiency dialysis until it is safe to begin DFO treatment. A clinician’s fear of using DFO, based entirely on problems that occurred with use of DFO in doses of 20 to 40 mg/kg, should give way to the timely use of DFO when it is needed using a "safe" dose of 5 mg/kg, followed by dialysis using a high-efficiency dialysis membrane. These precautions are designed to minimize any risk of side-effects of the DFO treatment.
With the great reduction of incidences of aluminum toxicity, large clinical trials to evaluate its treatment are not likely to occur or to be possible. Some nephrologists still believe that certain "low doses" of aluminum gels are indeed both safe and effective to control serum phosphorus levels. It would be well to establish long-term, prospective trials in such patients to assess the safety of the treatment in comparison to other nonaluminum-based phosphate binders. There is little doubt that aluminum-based phosphate binders are more potent and effective in binding dietary phosphate, in comparison to similar doses of other phosphate-binding agents.144
Very small and uncontrolled trials indicate that it is possible to give aluminum-based binders combined with very small doses of DFO (<1 mg/kg), and the investigators reported a slow, gradual reduction of plasma aluminum levels during such treatment.438 Others have shown that DFO in doses of 0.5 to 1.0 mg/kg increases the ultrafilterable (and hence the dialyzable) level of plasma aluminum in dialysis patients with elevated plasma aluminum levels.437 These data provide the background for a potential prospective trial that could test the safety and effectiveness of aluminum gels combined with repeated, very low doses of DFO in comparison to other nonaluminum-based phosphate binding agents.
Investigators with interest in aluminum toxicity and its treatment need to collect additional series and cases where the DFO is given in "low" doses of 5 mg/kg BW or even less. Recognizing whether cases of mucormycosis will be seen with such doses and use of high-efficiency dialyzers is also needed.
In a population with substantial numbers of patients with aluminum overload and minimal symptoms, controlled trials comparing total aluminum withdrawal with DFO treatment would be worthwhile to prove the advantage of DFO treatment over total withdrawal of exposure to aluminum.