This Guideline encompasses 2 parts: Guideline 8A, which deals with active vitamin D sterol therapy in CKD Stages 3 and 4, and Guideline 8B, which deals with CKD Stage 5.
GUIDELINE 8A. ACTIVE VITAMIN D THERAPY IN PATIENTS WITH STAGES 3 AND 4 CKD (ALGORITHM 2)
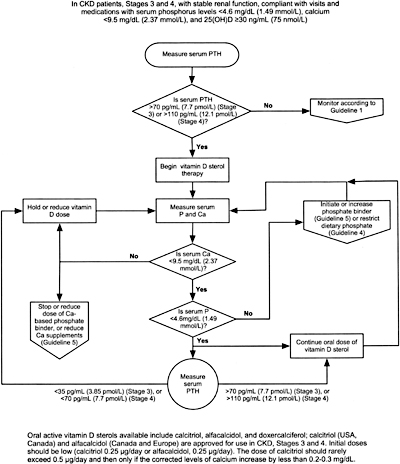
8A.1 In patients with CKD Stages 3 and 4, therapy with an active oral vitamin D sterol (calcitriol, alfacalcidol, or doxercalciferol) is indicated when serum levels of 25(OH)-vitamin D are >30 ng/mL (75 nmol/L), and plasma levels of intact PTH are above the target range for the CKD stage (see Table 15, Guideline 1). (EVIDENCE) Initial doses are provided in Table 27.
8A.1a Treatment with an active vitamin D sterol should be undertaken only in patients with serum levels of corrected total calcium <9.5 mg/dL (2.37 mmol/L) and serum phosphorus <4.6 mg/dL (1.49 mmol/L). (OPINION)
8A.1b Vitamin D sterols should not be prescribed for patients with rapidly worsening kidney function or those who are noncompliant with medications or follow-up. (OPINION)
8A.2 During therapy with vitamin D sterols, serum levels of calcium and phosphorus should be monitored at least every month after initiation of therapy for the first 3 months, then every 3 months thereafter. Plasma PTH levels should be measured at least every 3 months for 6 months, and every 3 months thereafter. (OPINION)
8A.3 Dosage adjustments for patients receiving active vitamin D sterol therapy should be made as follows:
8A.3a If plasma levels of intact PTH fall below the target range for the CKD stage (Table 15, Guideline 1), hold active vitamin D sterol therapy until plasma levels of intact PTH rise to above the target range, then resume treatment with the dose of active vitamin D sterol reduced by half. If the lowest daily dose of the active vitamin D sterol is being used, reduce to alternate-day dosing. (OPINION)
8A.3b If serum levels of corrected total calcium exceed 9.5 mg/dL (2.37 mmol/L), hold active vitamin D sterol therapy until serum calcium returns to < 9.5 mg/dL (2.37 mmol/L), then resume treatment at half the previous dose. If the lowest daily dose of the active vitamin D sterol is being used, reduce to alternate-day dosing. (OPINION)
8A.3c If serum levels of phosphorus rise to > 4.6 mg/dL (1.49 mmol/L), hold active vitamin D therapy, initiate or increase dose of phosphate binder until the levels of serum phosphorus fall to ≤ 4.6 mg/dL (1.49 mmol/L); then resume the prior dose of active vitamin D sterol. (OPINION)
In CKD patients with GFR below 60 mL/min/1.73 m2 (Stage 3), secondary hyperparathyroidism with elevated PTH levels is common and bone biopsies disclose hyperparathyroid bone disease in a large fraction of patients. The administration of small doses of the active vitamin D sterols, calcitriol and alfacalcidol, reduce the serum levels of intact PTH, improve bone histology, and lead to increased bone mineral density (BMD). With the use of low dosages, these effects occur with no evidence of worsening of kidney function; however, careful monitoring of serum levels of calcium, phosphorus and intact PTH is essential.
Algorithm 2. Management of CKD patients (Stages 3 and 4) with active Vitamin D sterols.
In CKD patients with GFR <60 mL/min/1.73 m2 (Stage 3), there is the appearance of secondary hyperparathyroidism with elevated serum levels of intact PTH.46,64,244,245 In such patients, bone biopsies show histomorphometric features of hyperparathyroid bone disease despite only modest elevations of intact PTH.19,246-248 Plasma levels of 1,25(OH)2D3 are either normal or in the lower range of normal,46,64,244,245 despite the elevated intact PTH levels and serum levels of phosphorus that are often in the low range of normal.10,188,249 Normal 1,25(OH)2D3 levels in the face of high levels of PTH are inappropriate and thus contribute to defective feedback suppression by 1,25(OH)2D3 of pre-PTH synthesis in the parathyroid glands with a resultant increased secretion of PTH.250
In controlled trials in patients with Stage 3 CKD, the administration of oral calcitriol, 0.25 µg/day and occasionally up to 0.5 µg/daily,246,251 or of alfacalcidol, 0.25 to 0.5 µg daily19 were associated with lowering of intact PTH levels,248,251 improvement of histological features of hyperparathyroid bone disease,246-248,252 or an increase of bone mineral density.251 Preliminary evidence also suggests that patients who had calcitriol therapy initiated when the creatinine clearance exceeded 30 mL/min/1.73 m2 (0.50 nmol/L/min/1.73 m2) had normal bone histology when they finally reached Stage 5 CKD and received a kidney transplant, while those whose treatment was started when kidney failure was more advanced were less likely to have normal bone histology when they reached end-stage kidney disease.253
There has been concern about the safety of the use of these vitamin D metabolites with regard to a possible adverse effect on kidney function. With the use of calcitriol in doses of 0.25 µg/day or less and doses of alfacalcidol that were generally below 0.5 µg/day, the progressive loss of kidney function did not differ from observations in placebo-treated or control patients.19, 246,248,252,254 In all CKD patients receiving vitamin D therapy, continued surveillance is needed, and hypercalcemia must be avoided. When calcitriol was given in doses of 0.5 µg/day or higher, reductions of creatinine clearance have been observed,255,256 although it is not certain that true GFR (inulin clearance) was affected.256,257 In CKD patients with serum levels of phosphorus >4.6 mg/dL (1.49 mmol/L), dietary phosphorus restriction and/or phosphate binders should be employed and the serum phosphorus normalized before initiation of treatment with an active vitamin D sterol.
Each of the placebo-controlled trials of CKD patients with GFR of 20 to 60 mL/min/1.73 m219,246,252 and 2 studies without a placebo-control group247,248 have shown evidence of hyperparathyroid bone disease in a high fraction of baseline "control" bone biopsies. Also, these abnormalities were common in the CKD patients recruited only on the basis of their impaired kidney function (reduced GFR or elevated serum creatinine levels) with the degree of elevation of pretreatment levels of intact PTH totally unknown.19,246,251,252 In each of the placebo-controlled trials, there was either no improvement or worsening19,246,252 of the features of hyperparathyroid bone disease in patients assigned to placebo therapy. Following treatment for 8, 12, or 24 months, an improvement of bone biopsy features was noted in the vitamin D-treated patients.19,246,247,252 Meta-analysis could not be done for these studies because 1 reported their data as mean ± SD,247 1 reported medians and ranges,246 and another reported the fractions of patients who showed improvement or worsening of various histological features on bone biopsy.19 Another study was excluded because the number of subjects was too small (n < 10).252
The safety of calcitriol or alfacalcidol in CKD with moderately reduced kidney function is a matter of concern; however, the data from the placebo-controlled studies show no reduction of kidney function compared to placebo in patients entered into these trials and using relatively low doses.19, 246,247,251,252 Should hypercalcemia develop during vitamin D treatment, particularly with higher doses, transient or even long-lasting deterioration of kidney function has been observed.258-260 With regard to the risk of producing "adynamic bone," the placebo-controlled trial that included the largest number of bone biopsies failed to show any increase in the appearance of adynamic bone disease following treatment with alfacalcidol.19
The available evidence is obtained from short-term studies and on a relatively small number of patients. Also, no data are available on the effect of the new vitamin D analogs, which are less hypercalcemic.
It appears that the active vitamin D sterols are useful in the treatment of secondary hyperparathyroidism and high-turnover bone disease in early stages of CKD. This provides a good therapeutic tool for the prevention and management of these 2 abnormalities in CKD patients, before these derangements advance and their treatment becomes more difficult.
Further trials with longer-term treatment (24 months or longer) in larger numbers of patients are needed to satisfy the concern about the safety of the therapy with vitamin D sterols. Trials with the newer vitamin D sterols which may be less calcemic will be of great interest. An ideal goal of such treatment would be to reduce serum levels of intact PTH with little or no change in serum levels of calcium. Studies should evaluate the effect on bone, in particular to ascertain whether improvement in bone mineral content or in histological features of hyperparathyroid bone disease could be achieved. Investigational Review Boards may feel that it is inappropriate to withhold vitamin D therapy in placebo-controlled studies. However, comparisons of newer vitamin D sterols with calcitriol, alfacalcidol, or even ergocalciferol, at 50,000 IU monthly, would be ideal. It is apparent that the ideal target for serum levels of intact PTH that should be sought are not established, and biopsy evaluations in such trials with correlations between intact PTH or whole PTH levels with new assays of PTH (see discussion in Guidelines 1 and 2) and skeletal findings would be ideal. Also, in the trials that have been published,19, 248 it would be useful if the data were reanalyzed to evaluate the relationship between serum levels of intact PTH and the degree of parathyroid bone disease found on biopsy in relation to the degree of impairment of kidney function.