ACE inhibitors and ARBs can be used safely in most patients with CKD.
11.1 ACE inhibitors and ARBs should be used at moderate to high doses, as used in clinical trials) (A).
11.2 ACE inhibitors and ARBs should be used as alternatives to each other, if the preferred class cannot be used (B).
11.3 ACE inhibitors and ARBs can be used in combination to lower blood pressure or reduce proteinuria (C).
11.4 Patients treated with ACE inhibitors or ARBs should be monitored for hypotension, decreased GFR, and hyperkalemia (A).
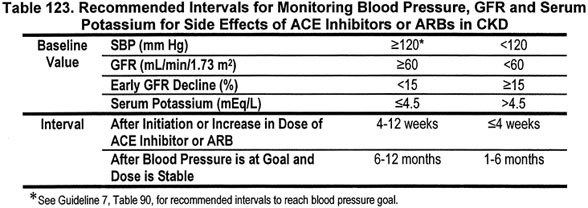
11.5 The interval for monitoring blood pressure, GFR, and serum potassium depends on baseline levels (Table 123) (B).
11.6 In most patients, the ACE inhibitor or ARB can be continued if:
11.6.a GFR decline over 4 months is <30% from baseline value (B);
11.7 ACE inhibitors and ARBs should not be used or used with caution in certain circumstances (Table 124).
Guidelines 8 and 9 recommend ACE inhibitors and ARBs as preferred agents for diabetic kidney disease and nondiabetic kidney diseases with proteinuria. In these diseases, they lower blood pressure, reduce proteinuria, slow the progression of kidney disease, and likely reduce CVD risk by mechanisms in addition to lowering blood pressure. In these types of CKD, ACE inhibitors and ARBs are recommended even in the absence of hypertension. ACE inhibitors and ARBS may also be used alone or in combination to reduce proteinuria in patients with or without hypertension.
The use of ACE inhibitors and ARBs may result in adverse effects, which are more common in CKD. The most common side-effects—early decrease in GFR, hypotension and hyperkalemia—can usually be managed without discontinuation of the agent. With careful monitoring of therapy, most patients can be treated with ACE inhibitors and ARBs, even at low levels of GFR.
The purpose of this guideline is to discuss general considerations of the use of ACE inhibitors and ARBs as preferred agents, to provide recommendations for initiation of therapy and dose escalation, and to provide recommendations for monitoring to enable early detection and management of side-effects.
The rationale is divided into three sections: (1) review of physiology and pharmacology; (2) recommendations for initiation and dose escalation; and (3) recommendations for monitoring and management of specific side-effects. The strength of evidence is graded only for the latter two sections. Within each set of recommendations, the definition and strength of evidence is reviewed.
Renin-Angiotensin System (Fig 54)
Fig 54. Physiology of the renin-angiotensin system and sites of action of ACE inhibitors and angiotensin-receptor blockers.
Renin, a proteolytic enzyme, is stored in the juxtaglomerular cells around the afferent glomerular arterioles. Under normal circumstances, it is released in response to stimuli such as reduced kidney blood flow or increased sympathetic tone. Renin acts on the substrate angiotensinogen to form angiotensin I. Angiotensin I undergoes transformation under the activity of several enzymes, including ACE and chymase, to angiotensin II. Angiotensin II has two major target receptors, AT1 and AT2. The stimulation of AT1 receptors, which appears to occur at a greater degree than stimulation of AT2 receptors, leads to significant efferent arteriolar constriction, which would have the effect of increasing the intraglomerular pressure head and maintaining filtration rate. AT2 receptor stimulation appears to produce antagonistic effects on efferent arterioles.
The main effects of angiotensin II are to constrict precapillary arterioles, leading to increased blood pressure, and to stimulate aldosterone release from the adrenal cortex, which in turn causes enhanced renal sodium retention and expansion of circulating blood volume.
In addition, angiotensin II has numerous effects within the kidney, including efferent arteriolar vasoconstriction, contraction of mesangial cells, stimulation of fibrogenic mediators, stimulation of free radical formation, and direct stimulation of tubular sodium reabsorption. Angiotension II also stimulates synthesis of aldosterone by the adrenal cortex. Overall, the net result of increased RAS activity in the kidney is increased glomerular pressure, increased glomerular permselectivity to macromolecules, activation of fibrogenesis, and increased oxidative stress.
Gene polymorphisms affecting the RAS. The variation in response to ACE inhibitors is potentially due to single nucleotide polymorphisms (SNPs) and insertion/deletion polymorphisms. SNPs occur in genes encoding ACE, angiotensinogen, and AT1 receptors. One explanation for conflicting data for ACE inhibitors is that the response may be influenced by a number of these polymorphisms in several genes. In addition, the occurrence of adverse effects may be due to the occurrence of polymorphisms, including cough and angioedema.
ACE inhibitors. ACE inhibitors are able to inhibit the ACE (Fig 54) and reduce the stimulation of both AT1 and AT2 receptors. However, ACE inhibitors have no effect on chymase, which may continue to lead to the production, on a smaller scale, of angiotensin II and thus stimulate AT1 and AT2 receptors. ACE also causes the degradation of bradykinin to inactive moieties. Thus, ACE inhibitors may cause an elevation in bradykinin levels. The consequences of ACE inhibitors therefore include a reduction in efferent arteriolar constriction, reduced aldosterone secretion, and enhanced kinin-induced peripheral vasodilation. Evidence also suggests that ACE inhibitors are able to promote the production of nitric oxide and vasodilator prostaglandins.
Angiotensin receptor blockers (ARBs). Current ARBs block only the AT1 receptor. This effect, if unopposed, could lead to an increased circulating level of angiotensin II and increased stimulation of AT2 receptors. Concerns have been raised about the adequacy of blockade of the RAS when using only an ACE inhibitor or an ARB alone. The function of ACE may be replaced by other enzymes (eg, chymase) leading to inadequate inhibition by ACE inhibitors and elevations in the levels of angiotensin II may compete with the AT1 receptor blockade provided by ARBs (Fig 54). Thus, combined ACE inhibitor/ARB therapy may be appropriate. Limited clinical data suggest that the combination may offer benefits in decreasing proteinuria and improving kidney perfusion.
Mechanism of Effects of ACE Inhibitors and ARBs to Slow Progression of CKD
As reviewed in Guidelines 8 and 9, ACE inhibitors and ARBs have a number of class effects that designate them as "preferred antihypertensive agents" for some types of CKD, even for patients without hypertension.
1. ACE inhibitors and ARBs reduce blood pressure. Hypertension accelerates the progression of kidney disease. The extent of blood pressure lowering correlates with the activity of the RAS. ACE inhibitors and ARBs have a greater antihypertensive effect in conditions in which blood pressure is maintained through stimulation of the RAS. By contrast, they have a lesser antihypertensive effect in conditions in which blood pressure is maintained through ECF volume overload, and concomitant suppression of the RAS. The lesser efficacy of ACE inhibitors and ARBs in African-Americans with essential hypertension compared to whites has been attributed to the higher prevalence of volume-mediated hypertension in African-Americans. CKD is associated with both stimulation of the RAS and ECF volume overload. ACE inhibitors and ARBs are generally effective antihypertensive agents in CKD; as single agents, they lower SBP and DBP by approximately 10 to 15 mm Hg and 5 to 10 mm Hg, respectively.
In experimental animals, ACE inhibitors and ARBs reduce intraglomerular pressure as well as systemic blood pressure, which contributes to their beneficial effect of slowing the progression of kidney disease. In controlled trials, the beneficial effect of ACE inhibitors and ARBs on the progression of kidney disease appears to be greater than would be expected based on their antihypertensive effects alone.
2. ACE inhibitors and ARBs reduce proteinuria. Proteinuria is associated with a faster progression of kidney disease. In general, reduction in proteinuria during antihypertensive therapy correlates with slowing the progression of kidney disease. In controlled trials in CKD, ACE inhibitors and ARBs reduce protein excretion by approximately 35% to 40%, which is greater than other antihypertensive agents, even when the effect of blood pressure reduction on urinary protein excretion has been taken into account (Figs 40 and 41). Calcium-channel blockers, on the other hand, have variable effects. The nondihydropyridine agents, such as verapamil and diltiazem, have significant antiproteinuric effects in diabetic—but not nondiabetic—kidney disease. The dihydropyridine agents, such as amlodipine and nifedipine, generally have no consistent effect on protein excretion. Recently, amlodipine was found to exacerbate protein loss in elderly African-American patients. Other agents, including diuretics, ß-blockers and a-blockers have not been shown to have a consistently significant effect on proteinuria. Comparison of the antiproteinuric effect of ACE inhibitors and ARBs is hampered by difficulty in establishing an equivalent dose.
In experimental animals, enhanced glomerular capillary pressure causes impaired glomerular permeability to proteins and permits excessive proteinuria. Reabsorption of filtered proteins can injure the interstitium of the kidney by activating intracellular events and the release of vasoactive and inflammatory mediators. Both ACE inhibitors and ARBs reduce the glomerular permeability barrier to proteins and limit proteinuria and filtered protein-dependent inflammatory signals. Some, but not all, controlled trials have shown that the beneficial effect of ACE inhibitors on the progression of kidney disease appears to be greater than expected due to their antiproteinuric effects.
3. ACE inhibitors and ARBs slow the progression of kidney disease by "class effect" mechanisms in addition to their antihypertensive and antiproteinuric effects. Possible mechanisms for these other effects of ACE inhibitors and ARBs include decrease in glomerular intracapillary pressure, reduction in permselectivity, alterations in the function of mesangial cells, and interfering with angiotensin-mediated generation of free radical formation.
Potentiation of ACE inhibitor and ARB effects by diuretics. ECF volume depletion is a potent stimulus of the RAS. As discussed in Guideline 12, diuretic therapy stimulates the RAS by reducing ECF volume, thereby enhancing the antihypertensive effect of ACE inhibitors and ARBs. As discussed in Guidelines 8 and 9, the combination diuretics with an ACE inhibitor or ARB therapy has had consistently beneficial effects on slowing the progression of kidney disease.
Therapeutic targets for ACE inhibitors and ARBs in CKD. Table 125 lists therapeutic targets for ACE inhibitors and ARBs in CKD and the guidelines in which the evidence for these targets is reviewed.
Adverse Effects of ACE Inhibitors and ARBs
The incidence of adverse effects from ACE inhibitors and ARBs is from 5% to 20%. Adverse effects of ACE inhibitors and ARBs may be classified into four main types (Table 126): (1) effects due to interfering with the activity of the renin-angiotensin-aldosterone system in the maintenance of blood pressure and serum potassium; (2) effects due to interfering with the activity of other enzymes and receptors; (3) allergic reactions; and (4) effects on the fetus.
Hypotension, early decrease in GFR, and hyperkalemia are dose-related adverse effects related to the mechanism of reduced angiotensin II levels, through either inhibition of ACE or blockade of the angiotensin II (AT1) receptor. These effects may be avoided by slow dose titration and managed by dose decreases or discontinuation.
Cough and angioneurotic edema are related to the effect of ACE inhibitors on other enzymes (Fig 55). The incidence of cough may be dose-related, and is not a true allergic effect. Angioneurotic edema is rare (<1%), but occurs more frequently in African-Americans than in Caucasians.
Fig 55. Physiology of side-effects of ACE inhibitors.
Allergic reactions include skin rash, neutropenia, or dysgeusia. Both ACE inhibitors and ARBs cause fetal abnormalities and are absolutely contraindicated in pregnancy in the second and third trimesters.
Strength of Evidence
ACE inhibitors and ARBs have not been tested in all types of CKD. Where tested, ACE inhibitors and ARBs have generally similar effects on blood pressure, urine protein excretion, and slowing the progression of kidney disease (Strong). Large, controlled trials have not been performed in all types of CKD, and there have been no direct comparisons of these two agents in any type of CKD. Their comparative efficacy remains unknown. It was the opinion of the Work Group that guidelines should reflect the strength of evidence for each agent in specific types of CKD. Thus, ACE inhibitors and ARBs were preferred for kidney disease with microalbuminuria due type 1 and type 2 diabetes; ACE inhibitors were preferred for kidney disease with macroalbuminuria due to type 1 diabetes and nondiabetic kidney disease with urine total protein-to-creatinine ratio ≥200 mg/g; and ARBs were considered preferred agents for kidney disease with macroalbuminuria due to type 2 diabetes. Both classes are preferred for reducing proteinuria. There does not appear to be a substantial difference among the ACE inhibitors, among the ARBs, or between ACE inhibitors or ARBs in regard to their antiproteinuric effects, although these comparisons are difficult due to differences in potency and administered dose.
Both classes of agents are effective in slowing the progression of experimentally induced CKD in animals. Both classes of agents are also effective in lowering blood pressure and reducing proteinuria in short-term studies in humans. Thus, the Work Group concluded that either class could be considered as a reasonable alternative to the preferred class, if the preferred class could not be used.
ACE inhibitors and ARBs in combination may be more effective than either agent alone (Weak). There are limited data regarding the potential benefits of combined therapy with ACE inhibitors and ARBs. Several small studies have shown an additive antiproteinuric effect when both ACE inhibitors and ARBs are used in combination; however, this finding has not been replicated in all studies. In one recent, large study of nondiabetic kidney disease464 combination therapy was more effective than either agent alone in slowing the decline in GFR. Until additional information is available, the use of either one class or the other preferentially is recommended, rather than both classes together.
Moderate to high doses of ACE inhibitors and ARBs have been associated with beneficial effects on kidney disease progression in controlled trials (Strong). Table 127 shows the normal dose range for of the ACEIs and ARBs that have been used in the major controlled trials. Unless adverse effects are noted, the use of similar or higher doses to achieve the therapeutic targets is recommended.
Therapy with ACE inhibitors or ARBs should be initiated at a moderate dose. The dose can be increased at 4- to 8-week intervals, with appropriate monitoring for side-effects. There is much variation in the duration of action of various ACE inhibitors. Those agents that have more than 50% of their peak effect still present at 24 hours can be prescribed once daily; those agents with less than 50% peak effect at 24 hours should be prescribed twice a day. The use of once-daily antihypertensive agents may be preferred due to the beneficial effects on patient compliance, and maintenance of normotension with fewer fluctuations in blood pressure compared to twice-daily antihypertensive agents.
Principles
Table 128 shows the general principles that should be followed when initiating treatment with ACE inhibitors or ARBs
Summary of Recommended Intervals for Monitoring of Side Effects
At initiation and increase in dose of ACE inhibitor or ARB, the levels of blood pressure, GFR, and serum potassium should be measured to establish a "baseline" or "new baseline." Frequency of monitoring depends on these baseline levels.
After initiation or change in dose of ACE inhibitor or ARB therapy (Table 129). Follow-up measurements should be made in approximately 4–12 weeks if SBP ≥120 mm Hg, GFR ≥60 mL/min/1.73 m2, change in GFR is <15%, and serum potassium ≤4.5 mEq/L. If SBP <120 mm Hg, GFR <60 mL/min/1.73 m2, change in GFR is ≥15%, or serum potassium >4.5 mEq/L, follow-up measurements should be at shorter intervals, and other interventions may be required, as detailed in the protocols below. In most cases, the ACE inhibitor or ARB should be continued, despite mild decreases in GFR and increases in serum potassium.
Patients should be counseled about allergic reactions. Patients should be instructed to contact their prescriber immediately if an allergic reaction occurs. ACE inhibitors and ARBs should be discontinued immediately if angioneurotic edema occurs. Patients with an ACE inhibitor-induced cough can be switched to an ARB.
ACE inhibitors and ARBs should not be used if there has been a documented pre-existing allergy to either (skin rash, neutropenia, agranulocytosis). If a patient has developed a cough while using an ACE inhibitor, an ARB can be prescribed. If a patient has developed angioneurotic edema while using an ACE inhibitor, an ARB can be prescribed with caution.
Women of child-bearing potential should be counseled about adverse effects on the fetus. Contraception should be maintained during therapy. Women who become pregnant while using an ACE inhibitor or ARB should discontinue taking the drug as soon as possible in the first trimester. There is no written recommendation for the duration of contraception following discontinuation of therapy, but members of the Work Group suggested continuing contraception for 1 to 3 months.
ACE inhibitors or ARBs should be prescribed with caution to women not practicing contraception. ACE inhibitors or ARB should be discontinued during pregnancy, and patients should be counseled about risks to the fetus. Women may breast-feed while taking an ACE inhibitor or an ARB.
After blood pressure is at goal and dose is stable (Table 130). Follow-up measurements should be made in approximately 6 to 12 months, if SBP ≥120 mm Hg, GFR ≥60 mL/min/1.73 m2, change in GFR is <15%, and serum potassium ≤4.5 mEq/L. If SBP <120 mm Hg, GFR <60 mL/min/1.73 m2, change in GFR is ≥15%, or serum potassium >4.5 mEq/L, follow-up measurements should be at shorter intervals. At each visit, providers should inquire about symptoms of allergic reactions, and in women of child-bearing potential, emphasize the importance of contraception.
Scope
The following sections of the rationale are divided into recommended protocols for management of specific adverse effects. Table 131 shows the percentage of side-effects reported in the literature reviewed by the Work Group. Entries in summary tables are grouped first by type of disease (diabetic kidney disease first), then by study design (randomized controlled trials first), then by study size (largest first).
Within each protocol, the definition and strength of evidence is reviewed. The Work Group considered the strength of evidence for monitoring to be "Moderately Strong." Based on their review of the literature and opinion, the Work Group developed more detailed recommendations for the timing of follow-up visits and measures to prevent and treat each adverse effect. Since these recommendations have not been tested in controlled trials, the strength of evidence was graded as "Weak." They are presented only to provide general guidance to the clinician.
Recommendations for Detection and Management of Hypotension
Definitions
For the purpose of this guideline, hypotension is defined as a decrease in SBP to <100 mm Hg. Table 132 lists causes of hypotension patients with CKD.
Transient abrupt decreases in blood pressure are more likely to occur after initiation of ACE inhibitors or ARBs, or after a dose escalation, than at stable dosage (Strong). Transient abrupt decreases in blood pressure occur in about 2.5% of patients. Starting therapy with moderate doses followed by slow titration of dose appears to reduce the incidence and severity of hypotension. The feasibility of increasing the dose depends on SBP and its change following the prior dose increase. Reduction in doses of other antihypertensive agents, or other medications that lower blood pressure may be required (Table 133). Clinicians should be cautious about lowering systolic blood pressure below 110 mm Hg. Patients treated with antihypertensive therapy with SBP <120 mm Hg should be monitored more frequently (Table 134).
Changes in management at the time of initiation or increase in dose of ACE inhibitors or ARBs and intervals for monitoring blood pressure depends on the baseline blood pressure (Tables 133 and 134) (Weak).
Definitions
An early decrease in GFR is defined as a decrease in GFR by more than 15% from baseline within 4 weeks after initiation of ACE inhibitor or ARB.
Strength of Evidence
Early decrease in GFR can be observed in CKD (Moderately Strong). RCTs have used various definitions for acute declines of kidney function, and the reported incidence varies from 4% to 17% (Table 131). Table 135 lists causes of acute decline in GFR in CKD. The most common causes are ECF volume depletion excessive doses of ACE inhibitors or ARBs and concomitant use of diuretics or NSAIDs. None of the studies reported outcomes of dose reduction in cases of abrupt declines in kidney function, but discontinuation of the ACE inhibitor or ARB because of this effect was reported in up to 9% of patients.
The frequency of follow-up measurement of GFR to detect early decrease in GFR and changes in management after initiation or increase in dose of ACE inhibitors or ARBs depend on the baseline GFR (Table 136) and the magnitude of early decrease in GFR (Table 137) (Weak). Table 136 shows frequency of follow-up measurements of GFR based on baseline GFR.
If GFR decreases by more than 30% over baseline, the dose of ACE inhibitor or ARB may need to be reduced, and the GFR reassessed frequently until kidney function has returned to baseline. Thereafter, the clinician should adjust doses and monitor GFR according to Table 137. If GFR does not return to baseline within the appropriate interval, ACE inhibitor or ARB should be discontinued and an alternative antihypertensive agent should be selected.
Definition
For the purposes of this guideline, hyperkalemia due to ACE inhibitors or ARBs is defined as an elevation of serum potassium concentration to >5.0 mEq/L. Therefore, a threshold serum potassium of 4.5 mEq/L is defined for measures to prevent hyperkalemia. Variation in local laboratory normal ranges may require local adjustment of the serum potassium cut-off levels given in this guideline.
Strength of Evidence
The incidence of hyperkalemia varies widely, depending on the definition (Strong). RCTs have used different definitions for hyperkalemia, such as a single measurement of serum potassium >5 mEq/L, >6 mEq/L, or when the use of a potassium-binding agent was required. Other definitions have been more exacting, such as a persistent increase of 0.5 mEq/L above baseline, or a single increase of 0.5 mEq/L regardless of baseline. Thus, reported incidence ranges from as low as <1% to as high as 62.5% of patients (Table 131). Some reports have suggested that the incidence may be higher with ACE inhibitors than ARBs.
Clinical factors in addition to therapy with ACE inhibitors or ARBs influence the risk of hyperkalemia (Strong). These factors include higher versus lower doses, and some studies have demonstrated positive correlations between elevations of serum potassium with reduced GFR and in the presence of either metabolic acidosis or heart failure (Table 138).
Concomitant medications are a modifiable cause of hyperkalemia in CKD (Strong). Table 139 shows medications that can raise serum potassium in CKD.
Excessive dietary intake is a modifiable cause of hyperkalemia (Strong). Table 140 shows common foods with a high potassium content (≥250 mg/100 g). Target potassium intake of a "low potassium diet" is ≤2 to 3 g/d (approximately 50 to 75 mEq/d).
Measures to lower serum potassium can be used to prevent or treat hyperkalemia due to ACE inhibitors or ARBs in CKD (Table 141) (Strong). Table 141 shows measures to lower serum potassium concentration in CKD. They include discontinuation or dose-reduction of medications that raise serum potassium, prescription of a low-potassium diet, loop diuretics, alkali replacement (if metabolic acidosis, serum bicarbonate concentration <21 mEq/L), or sodium polystyrene sulfonate (Kayexalate). These measures can be used to prevent hyperkalemia prior to initiation or increase in the dose of ACE inhibitors or ARBs as well as to treat hyperkalemia after initiation or increase in dose of ACE inhibitor or ARB. (Note: These are measures for outpatient management of mild-to-moderate elevations in serum potassium concentration. Rarely, ACE inhibitors or ARBs may cause severe hyperkalemia, requiring emergency management. The discussion of emergency management of severe hyperkalemia is beyond the scope of these guidelines.)
Measures to prevent and manage hyperkalemia should be based on baseline serum potassium (Table 142) (Weak). Table 142 shows measures to reduce serum potassium concentration to prevent hyperkalemia prior to initiation or increase in the dose of ACE inhibitors or ARBs. The table also specifies the frequency of follow-up measurements of serum potassium to detect hyperkalemia after initiation or increase in dose of an ACE inhibitor or ARB, according to the baseline serum potassium concentration. If hyperkalemia develops, reduce the dose of ACE inhibitor or ARB by 50% and reassess the serum potassium every 5 to 7 days until serum potassium has returned to baseline. If serum potassium does not return to baseline within 2 to 4 weeks, discontinue the ACE inhibitor or ARB and select an alternate antihypertensive agent.
Definition
Other adverse reactions discussed in this section include those related to the action of ACE inhibitors to inhibit the conversion of bradykinin to inactive metabolites (cough and angioedema) (Fig 54), and allergic reactions (dysgeusia, neutropenia, and agranulocytosis).
Strength of Evidence
Incidence of adverse reactions is shown in Table 125 and Table 131. There is the potential for cross-reactivity between ACE inhibitors and ARBs.
Cough (Moderately Strong). ACE inhibitor–induced cough may occur in about 10% to 20% of patients, but may be up to 40% in patients with CHF. Cough is more common in females, African-Americans, and Chinese. The accumulation of bradykinin is considered to be responsible for cough production. Since ARBs do not inhibit bradykinin degradation, ARBs are less likely to produce a cough than ACE inhibitors (Fig 54). The cough is dry, nonproductive, and persistent. The severity ranges from innocuous to debilitating. The onset varies from days to months after starting ACE inhibitor therapy, and regress within a few days of discontinuation.
Angioedema (Moderately Strong). Angioedema is rare (<1%), but may be life-threatening. Genetic polymorphisms may be a risk factor for angioedema and cough. Angioedema is more common in African-Americans than in whites. Facial edema requires drug withdrawal, while laryngeal involvement requires emergency care.
Allergic reactions (Moderately Strong). Skin reactions may occur in up to 5% to 10% of patients and may be dose-related. The higher incidence of some of these effects with captopril may be due to the presence of a sulfyhdryl group.
Definitions
Most ACE inhibitors and ARBs are classified as category C during the first trimester, and category D during the second and third trimesters. There are fewer data available for the ARB class, but the Work Group members have considered them to be similar in effect to ACE inhibitors.
Strength of Evidence
ACE inhibitors (Strong) and ARBs (Weak) have adverse effects on the fetus during the second and third trimesters of pregnancy. There appears to be little risk of fetal abnormalities from exposure to ACE inhibitors and ARBs during the first trimester, but they should be discontinued immediately after pregnancy is noted. However, during the second and third trimester, ACE inhibitors and ARBs can cause kidney and lung toxicity and skull hypoplasia. Thus, ACE inhibitors are absolutely avoided during the second and third trimesters. There is limited information about the effect of ARBs in the second and third trimesters. To avoid the risk of fetal abnormalities, the Work Group recommends not using ACE inhibitors or ARBs during pregnancy. ACE inhibitors and ARBs appear in breast milk, but there appears to be no adverse effects on development in the neonate or infant. Recommendations for use in women of child-bearing potential are given in Table 143.
Table 144 summarizes use of ACE inhibitors and ARBs in CKD.
Compared to the wealth of data on antihypertensive agents in the general population, there are few data that examine the use of antihypertensive agents in CKD, and fewer data on the adverse effects of those agents. Many of the randomized trials do not provide adequate definitions of the key adverse effects, such as hypotension, decreased kidney function, and hyperkalemia. Data concerning the allergic reactions and fetal abnormalities are derived primarily from observational studies. There are few comparative data either within groups of ACE inhibitors or ARBs, or among agents from different classes. It is unclear whether substitution of one agent from a different class will attenuate an adverse effect. Risk factors for adverse effects need clarification.
Broad educational efforts are necessary to increase the use of ACE inhibitors and ARBs in CKD. Table 144 contains a summary of important information about the use of ACE inhibitors and ARBs in CKD.
Systematic approaches are critical for appropriate monitoring of patients for adverse effects. These will include not only regular monitoring during routine office visits, but also methods for education of patients to enable self-monitoring. Self-monitoring by patients is especially important for the allergic reactions, which may become life-threatening. Ongoing education of patients will also be important for identification and diminution of risk factors for adverse effects, thereby potentially preventing adverse effect appearance.
Additional clinical studies are needed for the treatment of hypertension using ACE inhibitors and ARBs in patients with CKD due to glomerular diseases. More studies are required with co administration of ACE inhibitors and ARBs. The specific dose of ACE inhibitors or ARBs that confers the optimum renoprotective effect remains unclear.
Future controlled trials will need to compare the dose-response relationship of antihypertensive agents to their adverse effects in patients with CKD. Studies should also be designed to determine differences between agents of the same class and among agents from different classes. Epidemiological studies will need to determine risk factors for adverse event formation.