Diuretics are useful in the management of most patients with CKD. They reduce ECF volume; lower blood pressure; potentiate the effects of ACE inhibitors, ARBs, and other antihypertensive agents; and reduce the risk of CVD in CKD. Choice of diuretic agents depends on the level of GFR and need for reduction in ECF volume.
12.1 Most patients with CKD should be treated with a diuretic (A).
12.1.a Thiazide diuretics given once daily are recommended in patients with GFR ≥30 mL/min/1.73 m2 (CKD Stages 1-3) (A);
12.1.b Loop diuretics given once or twice daily are recommended in patients with GFR <30 mL/min/1.73 m2 (CKD Stages 4-5) (A);
12.1.c Loop diuretics given once or twice daily, in combination with thiazide diuretics, can be used for patients with ECF volume expansion and edema (A).
12.1.d Potassium-sparing diuretics should be used with caution:
12.1.d.i In patients with GFR <30 mL/min/1.73 m2 (CKD Stages 4-5) (A);
12.1.d.ii In patients receiving concomitant therapy with ACE inhibitors or ARBs (A);
12.1.d.iii In patients with additional risk factors for hyperkalemia (A).
12.2 Patients treated with diuretics should be monitored for:
12.2.a Volume depletion, manifest by hypotension or decreased GFR (A);
12.2.b Hypokalemia and other electrolyte abnormalities (A).
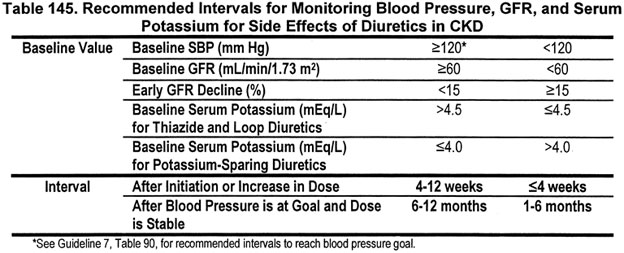
12.2.c The interval for monitoring depends on baseline values for blood pressure, GFR and serum potassium concentration (Table 145) (B).
12.3 Long-acting diuretics and combinations of diuretics with other antihypertensive agents should be considered to increase patient adherence (B).
Diuretics are generally necessary in CKD for control of extracellular fluid (ECF) volume expansion and for their associated effects on blood pressure. Based on the results of ALLHAT, JNC 7 recommends diuretics as preferred agents in the general population with essential hypertension to lower blood pressure and reduce CVD risk.5,5a Guidelines 8, 9, 10 recommend diuretics in combination with ACE inhibitors and ARBs in diabetic kidney disease and nondiabetic kidney disease with spot urine total protein to creatinine ratio of ≥200 mg/g, as preferred agents in nondiabetic kidney disease with spot urinetotal protein to creatinine ratio of <200 mg/g, and in combination with other agents in kidney disease in kidney transplant recipients.
Multiple diuretic classes are available for use in CKD including thiazides, loop diuretics, and potassium-sparing diuretics. Thiazide diuretics may lower blood pressure and reduce CVD risk by mechanisms in addition to reduction in ECF volume. Selection of diuretic agents depends on the level of GFR and need for reduction in ECF volume. Diuretic-related side-effects in CKD patients are similar to those observed with diuretic therapy in the general population, possibly differing in magnitude and frequency, which may relate to the typically higher diuretic doses employed in CKD.
The purpose of this guideline is to review general considerations in the use of diuretics; to make recommendations for initiation of therapy and dose escalation; and to make recommendations for monitoring therapy to enable early detection and management of side-effects.
The rationale is divided into three sections: (1) review of physiology and pharmacology, (2) recommendations for initiation and dose escalation, and (3) recommendations for monitoring and management of specific side-effects. Strength of evidence is graded only for the latter two sections. Within each set of recommendations, the definitions and strength of evidence will be reviewed.
A thorough application of the determinants of diuretic response is a prerequisite for the proper use of diuretics in CKD.
Sodium Retention in CKD
Sodium retention occurs when sodium intake exceeds sodium excretion and leads to ECF volume expansion. ECF volume expansion is common in CKD and is an important cause of hypertension (Table 146). In principle, the mechanism of decreased sodium excretion in CKD is reduced glomerular filtration of sodium, increased tubular reabsorption of sodium, or both. It is useful to think of two patterns of altered pathophysiology:
Sodium retention due to decreased filtered load. In principle, ECF volume expansion could lead to compensatory decrease in tubular reabsorption of sodium, re-establishment of the steady-state of sodium balance, with resultant hypertension, but without other manifestations of ECF volume expansion. It appears that tubular reabsorption is not truly appropriately suppressed. This is the most common pattern observed in CKD.530 Large increases in ECF volume may arise if sodium intake is very high or reduction in GFR is severe (for example, CKD Stage 4-5).
Sodium retention due to increased tubular reabsorption. Compensatory mechanisms may not be adequate, leading to large increases in ECF volume expansion with accompanying signs and symptoms. Conditions causing increased sodium reabsorption include nephrotic syndrome, heart failure, or cirrhosis. Drugs may also cause increased sodium reabsorption, including fludrocortisone (aldosterone), some estrogen compounds, and nonsteroidal anti-inflammatory agents.531 Patients with increased sodium reabsorption may not be in a steady state of sodium balance and are said to have a "tendency for sodium retention."
Use of Diuretics as Antihypertensive Agents
Diuretics act primarily by decreasing tubular sodium reabsorption, thereby increasing sodium excretion, reversing ECF volume expansion, and lowering blood pressure. Similarly, diuretic therapy can facilitate the response to other antihypertensive agents in CKD. Of course, reversal of ECF volume expansion depends on the balance of the diuretic effect on sodium excretion and ongoing sodium intake. Failure of diuretic therapy to lower blood pressure and restore ECF volume may be caused by excessive sodium intake or inadequate diuretic action.
Long-term therapy with thiazide diuretics may also reduce blood pressure by mechanisms other than reducing ECF volume. As a corollary, a blood pressure reduction with thiazide-type diuretics may occur even in the absence of a significant diuresis.
Fig 56. Determinants of diuretic response. Sodium excretion rate as a function of tubular delivery of diuretic. "A" represents pharmacokinetic determinants of diuretic response for an orally administered diuretic. The solid sigmoidal-shaped dose-response curve has three components: threshold (diuretic delivery rate sufficient to first produce a diuresis); efficiency (rate of delivery that produces an optimal response for any amount of diuretic entering the urine); and maximal response (urinary delivery of diuretic above which no additional diuretic response can occur). "B" represents altered pharmacodynamic determinants in "diuretic resistance," in which the normal simoidal-shaped curve is shifted downwards and to the right. Diuretic delivery necessary to achieve a threshold response can vary substantially in diuretic resistance.
Principles of Diuretic Action
Diuretic action is a coordinated process, which first relies on an adequate amount of the drug having reached its site of action, the renal tubule (Fig 56). When a diuretic is given intravenously there is no concern about bioavailability; alternatively, when administered orally, the rate and extent of absorption of a diuretic become important considerations in the pattern of the diuretic response. Once present in the circulation, a diuretic must gain entry into the renal tubule in a sufficient concentration to exceed the threshold for response; thereafter, there exists an optimal rate of drug delivery leading to and a rate of a maximal response (Fig 56, solid dose-response line). Additional diuretic delivery does not produce a greater response.526,527 The response is also dictated by a variety of other factors, including the "braking phenomenon." The braking phenomenon (postdiuretic sodium retention) describes avid sodium retention that can develop in response to a rapid diuresis, thereby limiting response to further doses of diuretics. The braking phenomenon may occur during either short-term or long-term therapy and is due to hemodynamic and neurohumoral changes produced by rapid diuresis. In CKD, the tubular secretory capacity for a diuretic is lowered, often in parallel with the reduction in GFR. Thus, higher blood levels are required to effect tubular delivery sufficient to prompt a diuresis.
Under normal circumstances, the aforementioned pattern of response is fairly predictable. In CKD, two different response patterns emerge. In the absence of a tendency to retain sodium, there is a normal sigmoidal-shaped dose-response relationship (Fig 56, solid dose-response line). In the presence of a tendency to retain sodium, the normal dose-response curve shifts rightward and downward, indicating a state of "diuretic resistance," in which a greater drug delivery rate is required to exceed the threshold for initiating a response, thereby attenuating the maximal response (Fig 56, hatched dose-response line).534,535
The plasma half-life of a diuretic determines its frequency of administration. The plasma half-life of loop diuretics is fairly short, with the exception of torsemide. This is of clinical importance in that once a short-acting loop diuretic has been administered, its effect disappears fairly quickly and well before the next diuretic dose, particularly if it is being given once daily. Shortly after the diuretic effect has waned, sodium reabsorption is increased, which may be sufficient to completely nullify the gain from the prior natriuresis. This rebound antinatriuretic effect (braking phenomenon) attenuates the normal dose-response relationship and can last several hours, thus limiting the efficacy of therapy. It can be overcome by administering multiple daily doses of the diuretic.535,536
Potentiation of Effects of Antihypertensive Agents by Diuretics
Diuretic therapy enhances the antihypertensive effect of most antihypertensive agents. The mechanism for this effect is that most antihypertensive agents stimulate renal tubular sodium reabsorption, thereby increasing ECF volume and blunting the antihypertensive effect. Diuretics interfere with sodium reabsorption, lower ECF volume, and potentiate the antihypertensive effect of the antihypertensive agent.
At the same time, reducing ECF volume activates neurohumoral pathways, especially the renin-angiotensin system, leading to vasoconstriction and increased systemic vascular resistance, which blunts the antihypertensive effect of diuretics. The combination of an ACE inhibitor or ARB with a diuretic is particularly effective in lowering blood pressure264 (Fig 57). The incremental reduction in blood pressure during combination therapy with either an ACE inhibitor or ARB and a diuretic is related to the degree of diuresis and therefore may be more significant when a more potent loop diuretic is being administered.
Fig 57. Rationale for combination of ARBs or ACE inhibitors with diuretics. Schematic depiction of additive antihypertensive effects of the combination of a diuretic and either an ACE inhibitor or an ARB. Volume loss produced by diuretic therapy activates the renin-angiotensin system, blunting the decline in blood pressure. Blockade by either ACE inhibitor or ARB increases the antihypertensive response.
Classes of Diuretics
There are three major classes of diuretics: thiazide diuretics, loop diuretics, and potassium-sparing diuretics (Table 147). Aldosterone antagonists act in the kidney as potassium-sparing diuretics. Their diuretic actions are discussed in Guideline 12. In addition, aldosterone antagonists act on mineralocorticoid receptors in the heart and blood vessels and at steroid receptors in other tissues. Their clinical effects are discussed in Guideline 7. A brief description of the pharmacodynamics and pharmacokinetics of each class is presented below.
Thiazide Diuretics
Thiazide diuretics act by inhibiting the apical Na+-Cl- cotransport system in the distal tubule.
Absorption. The absorption of thiazide diuretics is compound-specific and related to formulation characteristics. For example, certain formulations of metolazone are very slowly and erratically absorbed, while others are very rapidly and completely absorbed. Variation in absorption is less relevant when dosing has been ongoing for a sufficiently long enough time to have established a steady-state blood level.
Distribution. Total protein binding for thiazide diuretics is typically very high with differing values for the various compounds that make up this drug class. The Vd for thiazide diuretics is also compound-specific, with chlorthalidone and metolazone having the largest Vd, in part, since these drugs both distribute fairly heavily into red blood cells. For example, the Vd for chlorthalidone has been reported to range between 3 and 13 L/kg. Although the relevance of a large Vd remains to be determined, it may prolong the duration of effect.
Metabolism. The thiazide diuretics are variably metabolized. Some thiazide diuretics, such as bendroflumethiazide and indapamide, are primarily metabolized by the liver. Others, such as hydrochlorothiazide and metolazone, are metabolized by the kidney. The metabolism/excretion of thiazide diuretics has not routinely served as a determinant of compound selection.
Excretion. Thiazide diuretics are delivered to their luminal site of action by organic anion transporters in the straight segment of the proximal tubule, which is a consequence of their extensive protein binding. The intrinsic secretory capacity at this site ultimately determines the amount of drug delivered into the lumen of the proximal tubule and subsequently carried to its site of action in the distal tubule. Glomerular filtration plays an inconsequential role in thiazide diuretic entry into the urinary space, because of the considerable protein binding. An assortment of factors influence drug availability for tubular secretion, including tubular transport capacity (usually correlated to the level of GFR), the quantity of circulating drug availability for secretion (which relates to the absolute bioavailability of the compound), systemic hemodynamics, and the time course of drug delivery. Increasing the dose will provide sufficient systemic drug concentrations to increase tubular delivery in amounts necessary to produce a diuretic response.537
Loop Diuretics
Loop diuretics act by inhibiting the Na+-K+-2Cl- cotransporter in the thick ascending limb of the loop of Henle.
Absorption. The bioavailability of loop diuretics is not affected by CKD. On average, 50% of an orally administered dose of furosemide is absorbed, but the range can be from 10% to 100%.538 This wide range makes it a matter of clinical judgement as to how much furosemide will be absorbed in an individual patient—particularly in patients with heart failure—and large doses of furosemide may be required before the drug is deemed ineffective. Intravenous administration can be used to bypass decreased absorption. In contrast, absorption of the two other loop diuretics marketed in the United States, bumetanide and torsemide, is nearly complete, ranging from 80% to 100%.
Distribution. Total protein binding for furosemide ranges from 91% to 99%. Both torsemide and bumetanide are also heavily protein-bound. Loop diuretics are primarily bound to albumin, which is reduced in patients with uremia and the nephrotic syndrome. Protein binding can be reduced by up to 10% in patients with decreased GFR. The volume of distribution (Vd) is low for all loop diuretics and is in the order of 0.2 to 0.5 L/kg, though the Vd can increase somewhat in patients with nephrotic syndrome.
Metabolism. The loop diuretics are variably metabolized. Torsemide is approximately 80% cleared by the liver, a process which involves the cytochrome P450 system. Although active metabolites of torsemide are formed, they are not present in sufficient amounts to influence the overall diuretic pattern. Bumetanide is approximately 50% metabolized by the liver and its half-life does not appreciably change in kidney failure. Approximately 50% of a dose of furosemide is excreted unchanged; the remainder is conjugated to glucuronic acid in the kidney. Therefore, in patients with kidney failure, the plasma half-life of furosemide is prolonged because both urinary excretion and conjugation by the kidney are reduced. The liver is only responsible for about 10% of the metabolism of furosemide.
Excretion. Like thiazide diuretics, loop diuretics are delivered to their luminal site of action by organic anion transporters in the straight segment of the proximal tubule. The intrinsic secretory capacity at this site determines the amount of drug that is conveyed into the proximal tubule lumen and subsequently carried to its site of action at the loop of Henle. Tubular delivery of loop diuretics is reduced in CKD, but the relationship between the rate at which a loop diuretic is excreted and the response in CKD is similar to that in normal subjects. For example, in patients with a GFR of approximately 15 mL/min, only 10% to 20% of the amount of most loop diuretics is secreted into tubular fluid as in normal subjects.539
Potassium-Sparing Diuretics
There are two principal types of potassium-sparing diuretics, those that inhibit epithelial sodium channels (triamterene and amiloride) and those that inhibit mineralocorticoid receptors (aldosterone antagonists). For both types, the site of action is in the collecting tubule.
Absorption. The absorption of potassium-sparing diuretics is quite variable and, to a degree, formulation-dependent, particularly for triamterene. If a potassium-sparing diuretic is administered as a component of a fixed-dose antihypertensive preparation, its absorption may also be influenced by the compounding procedure for such combinations.540
Distribution. The total protein binding is low for amiloride and its Vd is in the order of 5 to 7 L/kg. The total protein binding for triamterene and its active sulfate ester metabolite are 60% to 70% and 90%, respectively. The total protein binding for spironolactone and its metabolites is about 90%.
Metabolism. Amiloride is predominantly cleared by the kidney and liver disease has little effect on its pharmacokinetics. Triamterene is extensively metabolized to a major hydroxytriamterene sulfate metabolite. Spironolactone is converted to several metabolites with the active compounds 7-a-thiomethylspirolactone and canrenone contributing a major portion of the activity profile of this compound.
Excretion. Amiloride undergoes significant excretion by the kidney, both by glomerular filtration and tubular secretion by the organic cation secretory pathway. The same pattern exists for triamterene and its active metabolite hydroxytriamterene sulfate. Amiloride and the combination of triamterene and its active metabolite are excreted in a limited fashion in the setting of kidney failure, which can substantially modify their pattern of activity. Spironolactone is metabolized by the liver; CKD does not influence its pharmacokinetic pattern in a meaningful way.
Resistance to Diuretics
Diuretic resistance reflects a failure to respond adequately to a diuretic regimen, due to alterations in pharmacokinetic determinants of tubular delivery or pharmacodynamic determinants of diuretic action in the tubular space.
Diuretic resistance in CKD may be due to an independent cause of increased tubular reabsorption of sodium, such as nephrotic syndrome, heart failure, cirrhosis, or use of non-steroidal anti-inflammatory agents. Apparent diuretic resistance in CKD may also be due to a high dietary intake of sodium. Estimating diuretic response with a 24-hour urine collection for determination of sodium excretion rate can assist in this diagnosis. A sodium excretion rate of >100 mmol/d suggests excessive dietary sodium intake.
Diuretic tolerance represents a pharmacodynamic alteration involving one of two processes: (1) a short-term process driven by ECF volume loss, wherein additional diuretic response is curtailed by the braking phenomenon; (2) a longer-term process where the continued exposure of the distal tubule to a high sodium load results in distal tubular cell hypertrophy and an excessive "recapture" of sodium delivered from more proximal locations. Distal tubular hypertrophy can be altered by combining a thiazide-type diuretic with a loop diuretic.541
Several mechanisms may contribute to diuretic resistance in nephrotic syndrome, including intratubular binding of loop diuretic by filtered albumin, decreased GFR, excessive tubular reabsorption of sodium at site proximal to the loop of Henle, or a disease state-related resistance to diuretic action at the cellular level. Treatment of nephrotic syndrome may require high doses of loop diuretics, a combination of loop and thiazide diuretics, or loop diuretics with albumin infusions.
Adverse Effects of Diuretics
Table 148 shows adverse effects due to diuretics. Electrolyte abnormalities of diuretic therapy are related to the duration and extent of diuresis. In patients without clinical manifestations of ECF volume expansion, large-volume diuresis is associated with ECF volume contraction, hypotension, and reduction in GFR, leading to symptoms and limiting further response to diuretics. Treatment includes discontinuation of diuretics and repletion with sodium chloride. In patients with ECF volume expansion, large-volume diuresis may occur without ECF volume contraction, hypotension, or reduced GFR, not causing symptoms and not limiting further diuretic response, potentially causing profound losses of potassium, hydrogen ion, and magnesium. Water retention may also occur, especially if water intake is excessive. The resulting clinical picture includes hypokalemia, metabolic alkalosis, hypomagnesemia, and hyponatremia. If ECF volume contraction ensues, the fall in GFR can reduce ongoing electrolyte losses but rarely does it "correct" the abnormalities in serum electrolytes that have already occurred. Treatment includes discontinuation of diuretics, repletion of urinary losses, and water restriction as necessary.
Dietary sodium intake is an important contributor to the adverse effects of diuretics. A high intake may prevent ECF volume depletion, thereby increasing urinary losses of potassium, magnesium, and calcium. Conversely, restriction of dietary intake of sodium can curtail these losses, but increase the risk of ECF volume depletion.542 Hypokalemia and metabolic alkalosis can be prevented by administration of potassium chloride or a potassium-sparing diuretic. Potassium chloride and potassium-sparing diuretics should be administered with caution in patients with GFR <60 mL/min/1.73 m2 (CKD Stages 3-5) because of the increased risk of hyperkalemia.
Strength of Evidence
The choice of diuretics will be dependent upon both the stage of CKD and the ECF volume overload in the patient. The dose ranges for commonly used diuretics are shown in Table 149.
Thiazide diuretics can be used in CKD Stages 1-3 (Strong). In the doses recommended in Table 149, thiazides are effective in generating a diuresis in patients with GFR greater than approximately 30 mL/min/1.73 m2.536,543 Whether their nondiuretic properties contribute to blood pressure control in CKD is not known.
Thiazide diuretics differ in their pharmacokinetics. Hydrochlorothiazide (HCTZ) should be started at a dose of 25 mg/d in Stage 1-3 CKD, with titration to 50 to 100 mg/d as necessary. Doses as low as 6.25 mg of HCTZ in fixed-dose combination antihypertensive products improve the blood pressure response to other nondiuretic antihypertensive medications such as ß-blockers and ACE inhibitors. Chlorthalidone was used in a dose range of 12.5 to 25 mg/d in ALLHAT. Chlorthalidone is longer-acting than HCTZ, resulting in better blood pressure control, but also a higher incidence of hypokalemia. Chlorthalidone may be effective at a lower GFR than HCTZ.
The basis for failure of thiazide diuretics to cause a diuresis at reduced GFR is their insufficient potency at the doses administered. If blood pressure control worsens or if volume expansion occurs as CKD progresses from Stages 1-3 to Stages 4-5 during treatment with a thiazide diuretic (either as monotherapy or as fixed-dose combination antihypertensive therapy), a loop diuretic should be substituted for the thiazide diuretic. However, if blood pressure remains controlled, and ECF volume expansion is not apparent, it may not be necessary to switch to a loop diuretic.
Unlike other thiazide diuretics, metolazone retains effectiveness at GFR below 30 mL/min/1.73 m2 at the doses recommended in Table 149. Metolazone is very poorly absorbed and this should be taken into account when both a dose and frequency of dosing are being determined. Metolazone (Zaroxylyn®) can be started at a dose of 2.5 to 5.0 mg daily and titrated to 10 to 20 mg daily, though these higher doses are seldom needed. Once metolazone has effected a diuresis, it can typically be dosed as infrequently as two to three times a week because of its very long half-life.535
Loop diuretics can be used in all stages of CKD (Strong). Loop diuretics are the most commonly used diuretics in CKD. In CKD Stages 4-5, furosemide should be started at a dose of 40 to 80 mg once daily with weekly titration upwards by 25% to 50% dependent upon the response and ECF volume. Once an effective dose has been established, the frequency with which it needs to be administered can be determined by specific clinical needs. In the absence of specific conditions causing increased sodium reabsorption (nephrotic syndrome, heart failure, or cirrhosis), a brisk diuretic response occurs in response to a loop diuretic with only nominal dose titration. The maximal natriuretic response occurs with intravenous bolus doses of 160 to 200 mg of furosemide, or the equivalent doses of bumetanide and torsemide, and little is accomplished by using larger doses.544 In patients with specific conditions causing increased sodium reabsorption, the response to a loop diuretic is attenuated in relationship to the severity of the underlying disease, and substantially higher doses of furosemide may be necessary to achieve a diuresis.
Loop diuretics are not as effective as thiazide diuretics in lowering blood pressure in CKD Stages 1-3. In CKD Stages 4-5, loop diuretic therapy is a useful adjunct therapy in the treatment of hypertension. Fixed-dose combination antihypertensive products containing a loop diuretic are currently not available in the United States.
Potassium-sparing diuretics are associated with an increased risk of hyperkalemia in CKD (Strong). Potassium-sparing diuretics must be used with caution in patients with CKD because of the risk of hyperkalemia. The risk of hyperkalemia is especially high in patients with GFR <30 mL/min/1.73 m2 receiving concomitant therapy with ACE inhibitors or ARBs or other conditions that raise serum potassium (Table 138).
Indications for potassium-sparing diuretics in CKD are persistent hypokalemia or resistant hypertension.545 If potassium-sparing diuretics are used in CKD, beginning with low doses and slow upwards titration, frequent monitoring of potassium levels is warranted.
Recently, aldosterone antagonists have been shown to be effective for the treatment of heart failure with systolic dysfunction. Patients with CKD Stages 1-3 were included in these studies, but not CKD Stages 4-5. Low initial doses are recommended with increases in dose frequency every 1 to 2 weeks. Maximal doses may not be possible due to hyperkalemia.
The presence of hyporeninemic hypoaldosteronism should be considered as a contraindication to the use of potassium-sparing diuretics. Fixed-dose combination products containing potassium-sparing diuretics and thiazide-type diuretics are available for most potassium-sparing agents. These have not been commonly used in CKD Stages 4-5 CKD because of the attendant risk of hyperkalemia.
Long-acting diuretics and combinations of diuretics with other antihypertensive agents should be used to increase patient adherence (Moderately Strong). Patients have been shown to have a higher rate of compliance with medications prescribed once per day compared to medications prescribed more than once per day (see Guideline 5). In addition, antihypertensive medications that have a half-life of greater than 24 hours are more likely to sustain a significant and sustained decrease in blood pressure over a 24-hour period, compared to antihypertensive medications with a half-life of less than 24 hours (see Guideline 7).
After therapeutic goals are reached, it may be more convenient to prescribe a fixed-dose, once-daily combination of antihypertensive agents.264 A variety of combination antihypertensive agents are available (Guideline 7, Table 103), which can be used to simplify the patient’s antihypertensive regimen. In patients with blood pressure 20 mm Hg or more above their goal blood pressure, a fixed dose combination with a diuretic is a good choice for initial therapy. For patients with a GFR >30 mL/min/1.73 m2, a preferred agent in combination with a thiazide diuretic may be an appropriate choice.
Principles
Table 150 shows the general principles that should be followed when initiating treatment with diuretics. The most common complication of diuretics is ECF volume depletion, which may lead to hypotension, a decrease in GFR, hypokalemia, and other electrolyte abnormalities (Table 151).
Summary of Frequency of Monitoring
At initiation and increase in dose of diuretics, the levels of blood pressure, GFR, and serum potassium should be measured to establish a "baseline" or "new baseline." The frequency of monitoring depends on these baseline levels (Tables 152 and 153). (Note: Frequency of follow-up for control of elevated blood pressure is reviewed in Guideline 7.)
Recommendations for Detection and Management of Volume Depletion
Definitions
Loss of 15% to 20% of ECF volume (2 to 3 L [kg] in a 70-kg adult without edema) is associated with symptoms and signs of volume depletion (Table 151). Smaller volume losses sometimes may be associated with the same manifestations, especially if blood pressure is low due to concomitant use of other antihypertensive agents.
Strength of Evidence
Hypotension and decreased GFR are complications of ECF volume contraction (Strong). The exact incidence of these adverse effects during diuretic therapy is poorly defined, since they are drug-specific and dose-dependent, and there is variability among patients.546 Hypotension and/or a transient and abrupt decrease in GFR are more common when diuretics are first coadministered with either an ACE inhibitor or an ARB, particularly those agents that are excreted by the kidney.547 Hypotension is also more frequent in patients with nephrotic syndrome, heart failure, or cirrhosis treated with large doses of diuretics. Other causes of ECF volume depletion in CKD are listed in Table 154.
These side-effects can be avoided by gradual titration of the dose and careful monitoring following institution of combined diuretic and ACE inhibitor or ARB therapy. Management consists of either decreasing the dose of the diuretic (and/or the ACE inhibitor or ARB) or by temporarily discontinuing the diuretic. In addition, transiently increasing dietary sodium intake will facilitate recovery. A more complete discussion of the monitoring and treatment strategies for hypotension and/or a fall in GFR in CKD patients is described in Guideline 11. The plan is relevant to diuretic-treated patients in that the CKD patient rarely receives diuretic therapy without also receiving either an ACE inhibitor or an ARB.
Recommendations for Detection and Management of Electrolyte Abnormalities
Definition
For the purposes of this guideline, hypokalemia is defined as serum potassium <3.5 mEq/L, hyperkalemia is defined as serum potassium >5.0 mEq/L, metabolic alkalosis is defined as a serum bicarbonate concentration greater than 30 mEq/L in patients without a disorder of ventilation, and hypomagnesemia is defined as serum magnesium <1.8 mEq/L (although the serum magnesium is a poor indicator of quantitative deficiency unless values below 1 mEq/L are present). Normal values for urinary calcium excretion are 100 to 250 mg/d in adult men and women.
Strength of Evidence
The most common side-effects with diuretics are disturbances in electrolyte balance (Strong). Hypokalemia or hyperkalemia, metabolic alkalosis, hypomagnesemia, and hypocalciuria or hypercalciuria (usually without changes in the serum calcium concentration) can either individually or collectively occur during diuretic therapy in CKD. As discussed earlier, most diuretic-related electrolyte side-effects are related to the dose of diuretics and the level of dietary sodium intake. In this regard, the higher the diuretic dose (and thereby the greater the duration of action), the greater the expected sodium excretion and losses of other electrolytes.548
Hypokalemia (Strong). A decline in serum potassium concentration is common with loop and/or thiazide-type diuretic administration, particularly in the elderly and in patients with clinical manifestations of ECF volume expansion. Hypokalemia with diuretic therapy is less common in patients with decreased GFR. Limiting dietary sodium intake can curtail urinary potassium losses and therein lessen the risk of hypokalemia. The risk of hypokalemia can also be attenuated, albeit in an unpredictable fashion, by coadministration of an ACE inhibitor or an ARB.549 In general, the risk of metabolic alkalosis as a consequence of diuretic therapy parallels the occurrence of hypokalemia. Hypokalemia in CKD is usually multifactorial (Tables 155 and 156).
Treatment of hypokalemia in CKD requires careful attention to treatment of the underlying cause. When the underlying cause is diuretic therapy, which must be continued, there are a variety of measures to raise serum potassium, including dietary modification, use of oral potassium supplements, and drugs, including potassium-sparing diuretics.
Foods rich in potassium are listed in Guideline 11, Table 140. Preparations of potassium for oral supplementation are listed in Table 157. Potassium chloride supplementation is required for patients with hypokalemia and metabolic alkalosis. Other preparations (alkalinizing salts) can be used in patients with hypokalemia without metabolic alkalosis.
ACE inhibitors, ARBs, beta-adrenergic blockers, and potassium-sparing diuretics can be used to raise the serum potassium. Potassium supplements and drugs to raise the serum potassium concentration should be used with caution in CKD, because the risk of "overcorrection" with the development of hyperkalemia is appreciably higher, particularly in CKD Stages 3 and 4.550 The frequency of follow-up is a function of the magnitude of hypokalemia. This should generally be weekly or sooner until a stable serum potassium value has been reached, at which time the frequency with which serum potassium is monitored can be decreased to monthly or bi-monthly to coincide with routine visits. Table 158 summarizes measures to raise serum potassium in CKD.
Hyperkalemia (Strong). Hyperkalemia can occur with potassium-sparing diuretics, and its occurrence signals the need for reduction in dose or discontinuation of therapy with a potassium-sparing agent.551 Causes and management of hyperkalemia in CKD are discussed in detail in Guideline 11.
Hypomagnesemia (Strong).
Total body magnesium deficiency is a common occurrence with loop diuretic therapy. It is difficult to identify on the basis of change in serum magnesium values alone. Variation in local laboratory normal ranges for magnesium values may require local adjustment of the serum magnesium value at which therapy is initiated. Urinary magnesium losses parallel those of potassium in loop diuretic-treated CKD patients; thus, deficiency in total body magnesium is likely in most diuretic-treated patients with hypokalemia.552 Magnesium deficiency increases tubular secretion of potassium and may cause or worsen hypokalemia. Firm evidence does not exist in support of total body magnesium deficiency contributing to complications of CKD other than interference in the release and action of parathyroid hormone and, therefore, a greater tendency to hypocalcemia. Alternatively, in the setting of CVD (for example, heart failure and/or supraventricular and ventricular arrhythmias), which often coexists with CKD, magnesium deficiency can precipitate arrhythmias, and correction or prevention of magnesium imbalance is imperative.553 A number of magnesium-containing salts and antacids are available for treatment (Table 159).553 Treatment is usually empirical and guided by changes in serum magnesium values. All potassium-sparing diuretics are also magnesium-sparing.
Disorders of calcium excretion (Strong). Diuretics affect systemic calcium balance by altering urine calcium excretion in a diverse fashion: thiazide-type drugs decrease urine calcium excretion, which is one aspect of how they cause hypercalcemia; loop diuretics increase urine calcium excretion and therefore cause hypocalcemia.554 The hypercalciuria seen with loop diuretics can accelerate the progression of secondary hyperparathyroidism.555 The maintenance of calcium-phosphate balance in the CKD patient is complex (see K/DOQI Clinical Practice Guidelines for Bone Disease),291 and clinicians should consider the effect of concomitant treatment with diuretics. Specific treatment measures that effectively decrease loop diuretic-related hypercalciuria include reduction in the diuretic dose, lowering sodium intake, and combining a loop diuretic with thiazide diuretics.
Recommendations for Management of Other Adverse Reactions
Definitions
Other adverse reactions discussed in this section include hyperuricemia and gout, allergic reactions, and effects on the fetus.
Strength of Evidence
Hyperuricemia and gout (Strong). ECF volume contraction increases tubular uric acid reabsorption, decreases uric acid excretion, and thereby raises the serum uric acid concentration, which can trigger a gouty attack. This is more common with loop diuretics than with thiazide diuretics though it can occur with the latter. Patients with a history of gout who are beginning diuretic therapy should be counseled about the risk of recurrent attack. Prophylactic therapy for gout, such as colchicine and/or allopurinol, should be considered for patients with frequent attacks.556
Allergic reactions (Moderately Strong). All diuretics with the exception of ethacrynic acid are sulfa derivatives. Recently, it has been determined that sulfonamide antibiotics and other sulfa derivates are not cross-reactive.557 However, patients with a history of allergy to sulfonamide antibiotics are at increased risk of allergic reactions to other drugs and should be counseled appropriately. Sulfa allergies can range from skin rashes to urticarial lesions to anaphylaxis, and can be immediate on first exposure to a sulfa-drug or gradual in onset over days to weeks. Immediate sulfa allergic responses and/or a prior history of Stevens-Johnson syndrome or toxic epidermal necrolysis should remain contraindications to administration of a sulfa-containing diuretic. However, other allergic responses are not absolute contraindications to the use of a sulfa-type diuretic. Thiazide and loop diuretics are also associated with photosensitivity and bulbous eruptions. The occurrence of such lesions should preclude the continued use of the offending agent.
Effects on the fetus (Weak). All diuretics with the exception of spironolactone are Pregnancy Class B (animal studies do not indicate a risk to the fetus and there are no controlled human studies, or animal studies do show an adverse effect on the fetus but well-controlled studies in pregnant women have failed to demonstrate a risk to the fetus). Spironolactone is Pregnancy Class C (studies have shown that the drug exerts animal teratogenic or embryocidal effects, but there are no controlled studies in women, or no studies are available in either animals or women). Spironolactone has anti-androgenic effects, including incomplete virilization of the male fetus. Diuretics should be used cautiously in pregnancy. Volume contraction and electrolyte disturbances as may occur with diuretic therapy should be avoided since they may adversely affect the fetus.558
Table 160 summarizes use of diuretics in CKD.
Limitations
There is limited information from controlled trials to guide diuretic dosing for blood pressure control. In addition, there are very limited data concerning the antiproteinuric effects of diuretics and the combination of diuretics and ACE inhibitors or ARBs. Moreover, the role of diuretic therapy in CKD progression when congestive heart failure coexists needs to be better clarified.
Diuretics are frequently used in CKD. However, educational efforts are necessary to enhance their use to achieve blood pressure control and to improve management of complications. Table 160 contains a summary of important information about the use of diuretics in CKD.
The clinician will need to develop a suitable system to monitor blood pressure and volume status if these are goals linked to the titration and/or the frequency of diuretic dosing. Education of the patient in the basics of home blood pressure monitoring as well as periodic measurement of body weight are important components of any such monitoring system. Instruction in dietary sodium restriction is an essential component of a treatment plan with a diuretic.
Additional clinical studies are needed to determine whether differences exist amongst the various loop diuretics in how each influences blood pressure independent of volume loss. Moreover, additional studies are needed to determine if the blood pressure-lowering response to a loop diuretic is better with ACE inhibitors or ARBs. Future controlled trials will need to explore the relationship between CKD progression and the electrolyte changes that accompany loop diuretic administration. Studies should also be designed to evaluate the impact on calcium-phosphate balance and the triggering of secondary hyperparathyroidism from the hypercalciuria produced by loop diuretic treatment. Finally, more studies are needed with combination loop and thiazide diuretic therapy to determine if this is a more effective and/or safer approach than high-dose therapy with a loop diuretic alone and to determine which is the best thiazide diuretic to combine with a loop diuretic.