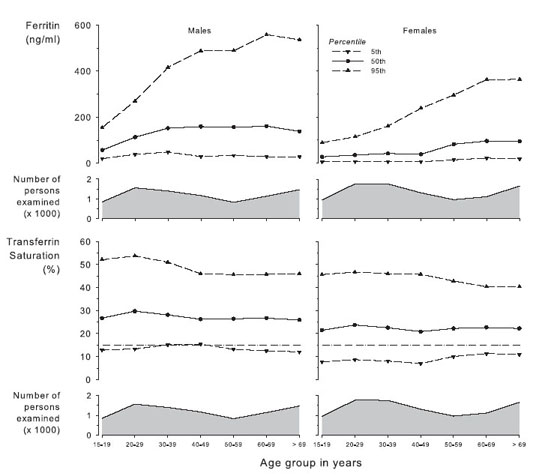
Fig 12. Distribution of ferritin and TSAT values by age group in males and females. Data are given as 5th, 50th, and 95th percentiles. Centers for Disease Control and Prevention; National Center for Health Statistics, 2005.40
Anemia in patients with CKD is not always caused by erythropoietin deficiency alone. Initial laboratory evaluation therefore is aimed at identifying other factors that may cause or contribute to anemia or lead to ESA hyporesponsiveness.
1.2.1 In the opinion of the Work Group, initial assessment of anemia should include the following tests:
1.2.1.1 A complete blood count (CBC) including—in addition to the Hb concentration—red blood cell indices (mean corpuscular hemoglobin [MCH], mean corpuscular volume [MCV], mean corpuscular hemoglobin concentration [MCHC]), white blood cell count, and differential and platelet count.
1.2.1.2 Absolute reticulocyte count.
1.2.1.3 Serum ferritin to assess iron stores.
1.2.1.4 Serum TSAT or content of Hb in reticulocytes (CHr) to assess adequacy of iron for erythropoiesis.
BACKGROUND
Although erythropoietin deficiency is common among patients with anemia and CKD, other potential causes and potentially contributing disorders should be identified or excluded. The recommended laboratory evaluation provides information regarding the degree and cause of anemia, activity of the erythroid and nonerythroid marrow, and assessment of iron stores and iron availability for erythropoiesis. In particular, clinicians should consider causes of anemia other than erythropoietin deficiency when: (1) severity of the anemia is disproportionate to the deficit in renal function; (2) there is evidence of iron deficiency, or (3) there is evidence of leukopenia or thrombocytopenia. An evaluation of the cause of anemia should precede initiation of ESA therapy. This guideline provides a framework for understanding the tests used in the initial evaluation of anemia and sheds light on the utility of test results in evaluating iron status.
RATIONALE
Initial Assessment of Anemia
The CBC provides information about the severity of anemia, adequacy of nutrients (including folate, vitamin B12, and iron), and adequacy of bone marrow function.
Severity of anemia is assessed best by measuring Hb concentration rather than Hct because Hb is a stable analyte that is measured directly. The Hb assay is standardized and is not influenced by differences in instrumentation. Conversely, Hct measurement is relatively unstable and lacks standardization. The Hct result is derived indirectly by automated analyzers and is instrumentation dependent.16 Analytical advantages of Hb level over Hct are described next.2
Although blood-sample storage conditions have no effect on Hb measurement, Hct increases with storage temperature and duration because stored red blood cells swell. The MCV from which the Hct is calculated (MCV × erythrocyte count) increases after 8 hours of storage at room temperature and after 24 hours when refrigerated.86 The degree of MCV increase after sample storage may erroneously elevate the resulting Hct value by as much as 2 to 4 Hct percentage points.87 Long sample shipping distances and sample transit times, as commonly required for US dialysis facilities, increase the risk for introducing error into Hct results.
Hyperglycemia is associated with an increase in MCV (but not Hb level) and therefore spuriously elevates the Hct result.88,89
The coefficient of variation for same-sample Hct is greater than that for Hb, largely because of variability in the number and size of erythrocytes counted to calculate the Hct.90 Within-run and between-run coefficients of variation in automated analyzer measurements of Hb are one half and one third those for Hct, respectively.91
For these reasons, Hct is an unacceptable test to evaluate anemia, and Hb should be the standard measure for assessing anemia.
Hb should be measured on standardized automated blood count analyzers in an accredited laboratory. In patients with non–dialysis-dependent (ND) CKD (ND-CKD) and patients with PD-dependent CKD (PD-CKD), the timing of the blood sample draw is not critical because plasma volume in these patients remains relatively constant. However, in patients with HD-dependent CKD (HD-CKD), interdialytic weight gain contributes to a dilutional decrease in Hb level, whereas intradialytic ultrafiltration promotes a contractional increase in Hb level. Thus, a sample obtained immediately before dialysis and during volume expansion underestimates the euvolemic Hb level, whereas a sample obtained immediately after dialysis overestimates the euvolemic Hb. In a study of 68 stable HD patients, mean predialysis versus postdialysis Hb levels were 10.5 ± 1.3 and 11.5 ± 1.3 g/dL, respectively.92 There was a strong linear inverse correlation between percentage of change in Hb and Hct values and percentage of change in body weight, indicating that increments in Hb and Hct values after dialysis were a direct function of the intradialytic variation in body weight and degree of ultrafiltration. Moreover, the predialysis Hb level measured after a long interdialytic period (3 days) was 0.5 to 0.6 g/dL less than the predialysis Hb level measured after a short interdialytic period (2 days). Among all pre-HD and post-HD Hb values, levels measured at the end of short intervals were closest to the mean Hb value of the week, derived from calculation of the area under the curve.93
Given the relationship between Hb level and interdialytic weight gain in patients with HD-CKD, midweek predialysis sampling theoretically is optimal. However, information that patient outcomes are affected by sampling day in patients with HD-CKD is lacking. Moreover, logistical considerations preclude sampling all patients with HD-CKD at midweek. Therefore, sampling for Hb determination should be performed before dialysis without specific reference to dialysis day. In the interpretation of results, consideration should be given to the potential effect of the patient’s volume status.
In addition to Hb, other reported results of the CBC may convey important clinical information. Deficiency of folate or vitamin B12 may lead to macrocytosis, whereas iron deficiency or inherited disorders of Hb formation (α- or β-thalassemia) may produce microcytosis. Macrocytosis with leukopenia or thrombocytopenia suggests a generalized disorder of hematopoiesis caused by toxins (eg, alcohol), nutritional deficit (vitamin B12 or folate deficiency), or myelodysplasia. When these findings are present, further diagnostic evaluation may be indicated. Hypochromia (low MCH) likely reflects longstanding iron-deficiency erythropoiesis.
In general, the anemia of CKD is normochromic and normocytic; that is, morphologically indistinguishable from the anemia of chronic disease. It characteristically is hypoproliferative: erythropoietic activity is low, consistent with insufficient erythropoietin stimulation. Proliferative activity is assessed by determination of the absolute reticulocyte count, the reticulocyte index, and the reticulocyte production index. The normal absolute reticulocyte count ranges from 40,000 to 50,000 cells/µL of whole blood. The reticulocyte index is calculated from the ratio of observed to normal reticulocyte count. Thus:
Reticulocyte index
= [observed absolute reticulocyte count]
÷ [normal absolute reticulocyte count]
Conversely, the reticulocyte production index corrects for the effects of erythropoietin-stimulated early release of reticulocytes from the bone marrow. Early release shortens the fraction of time reticulocytes mature in marrow and proportionally prolongs their maturation time in circulation. The result of early release is an increase in total reticulocyte count. However, that increase reflects the premature shift to the circulation, not increased erythroid production.94 Normal maturation time in circulation is 1 day. The expected maturation time, presuming a sufficient erythropoietin response to anemia, increases to 1.5 days at Hb values between 10 and 13 g/dL, 2 days at values between 7 and 10 g/dL, and 2.5 days at values between 3 and 7 g/dL. The reticulocyte production index is calculated by dividing the reticulocyte index by the expected maturation time. Thus:
Reticulocyte production index
= [reticulocyte index]
÷ [expected maturation time]
In an anemic patient, a reticulocyte production index greater than 3 is evidence of a normal proliferative response to anemia, whereas a production index of 2 or less is regarded as hypoproliferative, ie, little or no effective response. Although there is significant between-patient variability in absolute reticulocyte count, the test is sufficiently useful to serve its intended purpose as a semiquantitative marker of erythropoietic activity. The specific utility of the reticulocyte production index for the diagnosis and management of anemia in patients with CKD has not been evaluated.
By contrast, erythropoietin levels are not routinely useful in distinguishing erythropoietin deficiency from other causes of anemia in clinical settings.18,19,85,95,96
Evaluating Iron Status in Anemic Patients With CKD
Iron status test results reflect either the level of iron in tissue stores or the adequacy of iron for erythropoiesis. Serum ferritin level is the only available blood marker of storage iron. Tests that reflect adequacy of iron for erythropoiesis include TSAT, MCV, and MCH and the related indices, percentage of hypochromic red blood cells (PHRC) and content of Hb in reticulocytes (CHr). Because long sample transport and storage times spuriously elevate PHRC results, the PHRC test is not suitable for routine use in many patient-care settings. MCV and MCH decrease to less than normal range only after long-standing iron deficiency. Thus, timely and reliable assessment of the adequacy of iron supply for erythropoiesis generally requires results of either TSAT or, when available, CHr. We further recommend testing Hb, ferritin, and TSAT or CHr together because the combination provides important insight into external iron balance and internal iron distribution. The distribution of ferritin and TSAT by age and sex in the general population is shown in Fig 12.

Fig 12. Distribution of ferritin and TSAT values by age group in males and females. Data are given as 5th, 50th, and 95th percentiles. Centers for Disease Control and Prevention; National Center for Health Statistics, 2005.40
Testing iron status before treating anemia in patients with CKD serves 2 purposes: to assess the potential contribution of iron deficiency to anemia and determine whether further evaluation for sources of gastrointestinal (GI) bleeding is needed. Iron status testing is poorly suited to serve a third purpose, the prediction of responsiveness to iron therapy, because no single iron test or combination of tests discriminates iron-responsive from iron-unresponsive patients and because patients respond to intravenous (IV) iron therapy even when iron status results are substantially greater than the range associated with evidence of iron deficiency (Guideline 3.2).
The potential contribution of iron deficiency to anemia is demonstrated best by examining the relationship between iron status test results and Hb difference from a reference value. Not surprisingly, the nature of the relationship depends on whether the test measures iron stores or iron adequacy for erythropoiesis.
The relationship between measures of iron stores (serum ferritin) and Hb difference from reference is shown in Fig 13. In patients with ND-CKD, Hb values do not decrease to less than reference level except at the lowest ferritin levels (<25 ng/mL in males and <11 ng/mL in females). These ferritin results closely approximate the fifth percentile of serum ferritin values in a randomly selected population (males ≥ 20 years, 33 ng/mL; females ≥ 60 years, 15 ng/mL; US Renal Data System [USRDS] special data request, NHANES 1999 to 2002 data set) and likely represent sex-specific thresholds at which iron stores are exhausted in patients with ND-CKD.
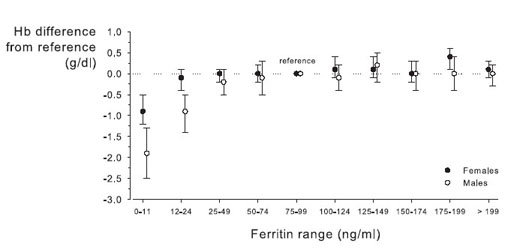
Fig 13. Distribution of Hb levels by ferritin range in patients with CKD. Results shown as mean 95% ± CI for parameter estimates. Reprinted with permission.26
In patients with HD-CKD, the relationship between serum ferritin and Hb values is less clear, probably because the relationship between ferritin level and iron stores may be disturbed. In relatively healthy patients with HD-CKD before widespread use of IV iron therapy, the finding of a ferritin level less than 50 ng/mL was not uncommon97 and was associated with absent bone marrow iron in approximately 80% of patients.98 However, in patients with a greater disease burden, absent iron stores may still be found at ferritin levels approaching or even exceeding 200 ng/mL.99
The relationship between adequacy of iron supply for erythropoiesis (from TSAT) and Hb difference from reference in patients with ND-CKD is shown in Fig 14. The relationship between TSAT and Hb values is continuous and relatively linear throughout the encountered range of TSAT values (Fig 14). There is no obvious threshold or cutoff value of TSAT below which the prevalence of anemia is sharply higher or the level of Hb is sharply lower. Hb values are significantly lower than reference (ie, Hb results seen in patients with a TSAT of 20% to 29%) among both males and females only when TSAT is less than 16%. By comparison, analysis of TSAT values in a randomly selected survey population showed that the fifth percentile of TSAT results in males 20 years and older is 12.5%, and that in females 60 years and older is 10.3% (USRDS special data request, NHANES 1999 to 2002 data set).
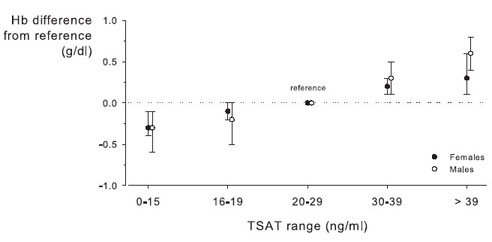
Fig 14. Distribution of Hb levels by TSAT range in patients with CKD. Results shown as mean ± 95% CI for parameter estimates. Reprinted with permission.26
In short, in patients with ND-CKD undergoing evaluation for anemia, ferritin levels less than 25 ng/mL in males and less than 12 ng/mL in females suggest that storage-iron depletion is contributing to anemia. Serum ferritin level is less reliable in the evaluation of iron stores in patients with HD-CKD than in those with ND-CKD. Iron-deficiency erythropoiesis is most likely to contribute to anemia when TSAT results are less than 16%. However, the clinical utility of TSAT is impaired by the absence of a diagnostic threshold.
Although iron status tests provide reasonable markers to detect iron deficits, there is little information to guide what action should be taken in response, particularly in regard to further diagnostic GI workup. In the absence of menstrual bleeding, iron depletion and iron deficiency result from blood loss from the GI tract. GI pathological states—including ulcer disease, colonic polyps, colorectal carcinoma, Helicobacter pylori positivity, and sprue—are common among iron-deficient patients referred for esophagogastroduodenoscopy and colonoscopy.100-103
Testing for occult blood in stool is not recommended as part of the evaluation of iron-deficient patients.104,105 As a screening test for colorectal carcinoma or precancerous colonic polyps, stool occult blood testing commonly is performed, but a high false-negative result rate renders it relatively unreliable.106 However, in a patient with iron deficiency, the test provides no useful information because a positive stool test result only confirms a diagnosis that the blood test results signifying iron deficiency have already established (GI blood loss), while a negative stool test result can only be falsely reassuring.
In patients with CKD, occult blood in stool was found in 6% to 7% of patients with HD-CKD and PD-CKD and nearly 20% of patients with ND-CKD.107 Among patients with heme-positive stools, follow-up endoscopy identifies GI pathological states (predominantly gastric and duodenal) in approximately 60% of patients. However, because the likelihood of finding GI lesions in patients with CKD with and without heme-negative stools has not been examined, critical information to establish the sensitivity and specificity of occult blood testing in the target population is lacking. Moreover, there is no information comparing the clinical utility of occult blood testing with that of endoscopy in the relevant patient population with iron deficiency, anemia, and CKD or in any target population with iron deficiency. Finally, there is no evidence that either endoscopy or other additional GI imaging improves patient outcomes when routinely conducted for the evaluation of iron deficiency. Thus, although colonoscopy can be part of an age-appropriate cancer screening and esophagogastroduodenoscopy should be strongly considered if iron deficiency is confirmed in an anemic patient with CKD, there is insufficient evidence to recommend their routine use for anemia evaluation.
In conclusion, although epidemiological evidence yields a consistent definition of iron depletion (ferritin < 25 ng/dL) and iron deficiency erythropoiesis (TSAT < 16%), in the absence of sufficient evidence that demonstrates therapeutic consequences based in these threshold values and in the presence of information that therapeutic intervention should be guided by other factors (see Guideline 3.2), these definitions lack specific recommendations for action and thereby fall short of constituting a guideline statement or CPR.
Further Evaluation
These tests are recommended only for the the initial evaluation of anemia. Should initial evaluation yield evidence for disorders other than erythropoietin deficiency or iron deficiency, further evaluation is warranted.