CPG AND CPR 3.2. USING IRON AGENTS
Anemia therapy in patients with CKD requires effective use of iron agents, guided by appropriate testing of iron status. Efficacy of iron therapy appears not to be limited to patients with evidence of iron deficiency. (See Guideline 1.2 for diagnosis of iron deficiency.) Thus, the goals of iron therapy are to avoid storage iron depletion, prevent iron-deficient erythropoiesis, and achieve and maintain target Hb levels.
3.2.1 Frequency of iron status tests:
In the opinion of the Work Group, iron status tests should be performed as follows:3.2.1.1 Every month during initial ESA treatment.
3.2.1.2 At least every 3 months during stable ESA treatment or in patients with HD-CKD not treated with an ESA.3.2.2 Interpretation of iron status tests:
In the opinion of the Work Group, results of iron status tests, Hb, and ESA dose should be interpreted together to guide iron therapy.3.2.3 Targets of iron therapy:
In the opinion of the Work Group, sufficient iron should be administered to generally maintain the following indices of iron status during ESA treatment:3.2.3.1 HD-CKD:
• Serum ferritin >200 ng/mL
AND
• TSAT >20%, or CHr >29 pg/cell.
3.2.3.2 ND-CKD and PD-CKD:
• Serum ferritin >100 ng/mL
AND
• TSAT >20%.3.2.4 Upper level of ferritin:
In the opinion of the Work Group, there is insufficient evidence to recommend routine administration of IV iron if serum ferritin level is greater than 500 ng/mL. When ferritin level is greater than 500 ng/mL, decisions regarding IV iron administration should weigh ESA responsiveness, Hb and TSAT level, and the patient's clinical status.3.2.5 Route of administration:
3.2.5.1 The preferred route of administration is IV in patients with HD-CKD. (STRONG RECOMMENDATION) 3.2.5.2 In the opinion of the Work Group, the route of iron administration can be either IV or oral in patients with ND-CKD or PD-CKD.
3.2.6 Hypersensitivity reactions:
In the opinion of the Work Group, resuscitative medication and personnel trained to evaluate and resuscitate anaphylaxis should be available whenever a dose of iron dextran is administered.
The goal of iron therapy in a patient with anemia and CKD is to achieve and maintain a target-range Hb level. Iron agents may serve as primary therapy for selected patients (particularly those with ND-CKD) or as adjuvant therapy for those also undergoing treatment with an ESA. Administered as adjuvants to ESAs, iron agents prevent iron deficiency and serve to minimize the dose of ESA needed to achieve target-range Hb levels. Designing appropriate iron therapy for patients with CKD-associated anemia requires an understanding of the interpretation of iron status test results, the therapeutic significance of iron status levels, and the therapeutic and safety limits of iron administration. Selecting a route of administration, dose, and class of iron agent requires an understanding of the efficacy, safety, and tolerability of available iron therapeutics. The stage of CKD and treatment setting (HD-CKD and PD-CKD) deserve consideration not only for practical reasons, but also because the quantity, quality, and strength of evidence vary among patients with ND-CKD, HD-CKD, and PD-CKD.
Frequency of Iron Status Tests Should Be Every 1 to 3 Months
The purpose of iron status testing is to either evaluate anemia or guide the use of iron agents to achieve and maintain targets of iron therapy during anemia management. Use and interpretation of iron status tests in the initial evaluation of anemia are described in Guideline 1.2.
Frequency of iron status testing during anemia management should be determined by the patient's Hb level relative to the target range and by the likelihood of blood loss. Initiation of ESA therapy, correction of a less-than-target Hb level during ongoing ESA therapy, bleeding, and surgical procedures each are likely to promote depletion of iron stores and thereby may call for increased frequency of iron status testing. Similarly, iron status testing is appropriate after treatment with a course of IV iron, to monitor safety and adequacy of therapy, and in a patient with hyporesponsiveness to ESA.
Clinical settings in which more frequent iron testing may be necessary include the following:
Interpretation of Iron Status Test Results Should Incorporate Hb and ESA Dose
In a patient undergoing ESA therapy, interpretation of the results of iron status tests should incorporate consideration of the Hb level and ESA dose. Together, these results provide information important to medical decision making because they elucidate the status of both external iron balance (net loss or gain of iron) and internal iron balance (disposition of iron between stores and circulating red blood corpuscles). For example, a decreasing ferritin level in the presence of a stable or decreasing Hb level may signify external iron loss (GI bleeding or, in patients with HD-CKD, dialysis-associated blood loss). In this case, iron therapy may be indicated to replace iron deficits. Conversely, a decreasing ferritin level in the presence of an increasing Hb level signifies an internal shift in iron from stores to Hb, as would be expected in a patient responding to ESA therapy. If iron status remains within the target range, additional iron administration may not be required. If the Hb level is greater than the target range and is being corrected by decreasing the ESA dose, ferritin level may increase as the Hb level corrects. Again, no iron may be needed. Finally, an increase in ferritin level accompanied by a decrease in TSAT suggests inflammation-mediated reticuloendothelial blockade and may be accompanied by a decrease in Hb level and increase in ESA dose. Whether and to what extent iron administration is effective and safe in this setting remains unresolved.
In short, medical decision making in using iron agents—including initiation of iron therapy, choice of route of iron administration, and determination of dose and duration of iron therapy—should be guided by results of iron status tests taken together with Hb levels and ESA dose, combined with a trend analysis of how each has changed over time.
Targets of Iron Therapy
In this section, we distinguish the therapeutic use of iron status tests from their diagnostic use. As diagnostic indices, iron status tests are helpful in determining the likelihood that iron deficiency is contributing to a low Hb level in patients with anemia and CKD (Guideline 1.2). In all patients with CKD, as in non-CKD patients, evidence of iron depletion and iron deficiency should prompt consideration of blood loss, and further evaluation may be needed (Guideline 1.2).
By contrast the statement Targets of iron therapy refers to results of iron status tests as treatment targets for the safe and effective use of iron agents. In patients receiving ESA therapy, iron status treatment targets serve to minimize the ESA doses required to maintain target-range Hb levels. In patients not receiving ESA therapy, iron status treatment targets serve to maximize the Hb level and minimize the need to initiate ESA therapy to achieve target-range Hb levels. Targets of iron therapy reflect the treat-to-target goals for the use of iron agents in patients with HD-CKD, ND-CKD, and PD-CKD with anemia.
Iron Targets in Patients With HD-CKD
Evidence to support the development of an iron target guideline in patients with HD-CKD is drawn from tissue iron studies (semiquantitative iron in bone marrow and quantitative iron in liver), iron challenge tests (response to IV iron administration), nonrandomized trials (efficacy of single-target IV iron intervention), and RCTs (comparing efficacy of 2 different iron targets). No iron intervention trial has been sufficiently powered to assess safety. Therefore, the Work Group sought to recommend iron targets that balance efficacy with assumptions regarding safety.
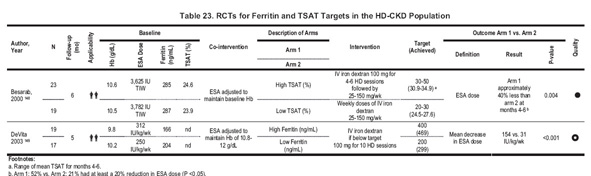
Serum ferritin level greater than 200 ng/mL. Earlier versions of KDOQI guidelines recommended a target ferritin level greater than 100 ng/mL. Two RCTs provide comparative information on iron status targets for IV iron therapy (Table 23). In 1 RCT, patients with HD-CKD randomized to maintain TSAT at 20% to 30% achieved a mean serum ferritin level of 297 ng/mL compared with those randomized to a TSAT of 30% to 50%, who achieved 730 ng/mL.148 Patients in the higher TSAT target group showed a mean 40% reduction in ESA dose compared with those in the lower group. However, the trial was small; iron status targets were defined by TSAT, not ferritin level; and ferritin level in the high-target TSAT group increased throughout the trial and had not reached a steady state by the end of study. In the second trial, patients with HD-CKD assigned to a 200-ng/mL ferritin target compared with those assigned to a 400-ng/mL target achieved mean ferritin levels of 299 compared with 469 ng/mL, respectively. Patients in the higher serum ferritin group had final ESA doses 28% lower than those in the lower ferritin group.149
Other available evidence reflects the likelihood of responsiveness to IV iron, but affords little information on the comparative safety and efficacy of iron status targets. Patients with high baseline ferritin levels (mean, 930 ng/mL) were administered IV iron if TSAT was less than 50% and ferritin level was less than 1,000 ng/mL. After receiving an average IV iron dose of 38 mg per week for 12 months, patients showed an increase in ferritin (mean, 1,383 ng/mL) and TSAT (from 27% to 36%) values and a 25% ESA dose decrease.150 Bone marrow examination in patients with HD-CKD may reveal little or no stainable iron in patients with ferritin levels greater than 100 ng/mL,98 particularly in patients with evidence of inflammation.99 It has been reported that IV iron challenge is associated with a positive response (an increase in reticulocyte or Hb values or a decrease in ESA dose) in many patients with serum ferritin levels greater than 100 ng/mL (Table 24). 151-156
Taken together, the evidence suggests that greater efficacy may be achieved if the lower limit of the ferritin target were greater than the 200 ng/mL threshold. However, given the absence of reliable safety information in clinical trials, uncertainty about the relevance of results from uncontrolled trials, bone marrow iron examination, IV iron challenge studies to iron treatment targets, and the concern for the risk for iron overload, the statement that serum ferritin levels generally should be maintained at greater than 200 ng/mL reflects acceptable—if not maximal—efficacy and a cautious consideration for patient safety.
TSAT greater than 20%. The recommended treatment target of TSAT is 20%, similar to previous KDOQI recommendations. In a single RCT comparing higher to lower TSAT targets, patients randomized to a target TSAT of 30% to 50% demonstrated a 40% reduction in ESA dose compared with those assigned to a target of 20% to 30% (Table 23).148 As is the situation for serum ferritin level, other sources of evidence are indirect and of uncertain value in setting TSAT targets. In an uncontrolled prospective trial described previously, patients with high baseline ferritin levels (mean, 930 ng/mL) were administered IV iron if TSAT was less than 50% and ferritin level was less than 1,000 ng/mL. After receiving an average IV iron dose of 38 mg/wk for 12 months, patients showed an increase in ferritin (mean, 1,383 ng/mL) and TSAT (from 27% to 36%) values and a 25% ESA dose decrease.150 Patients with a TSAT of 20% or greater may still show absent bone marrow iron (Table 25) or respond to IV iron challenge (Table 26). 98, 99,151-156 The statement that TSAT generally should be maintained at greater than 20% therefore reflects acceptable, if not maximal, efficacy and a cautious consideration for patient safety.
CHr greater than 29 pg/cell. Evidence to support a treatment target for CHr is drawn from 2 RCTs comparing the use of CHr with TSAT as an iron target. In the first, patients were assigned to receive IV iron for a CHr less than 29 pg/cell. A control group received IV iron for either TSAT less than 20% or ferritin level less than 100 ng/mL. Ferritin and TSAT were followed up in both groups, and IV iron was withheld in any patient with a TSAT greater than 50% or ferritin level greater than 800 ng/mL. Patients assigned to CHr targets showed a lower cost of anemia treatment and a decrease in IV iron use compared with those assigned to TSAT/ferritin targets.157
In the second RCT, patients assigned to a CHr treatment target of 32.5 pg/cell or greater showed no change in ESA dose, whereas those assigned to a TSAT treatment target of 20% or greater showed a 38% decrease in ESA dose. However, between-group ESA differences did not achieve significance.158 Functional iron studies have shown that the clinical utility of CHr as a baseline index of response to IV iron challenge varies (Table 27). 151,153-155,159 Results of CHr also may be instrument dependent. The current recommendation of CHr greater than 29 pg/cell is based on the Advia 120 instrument (Bayer Diagnostics Inc).
Other iron status tests have been studied, including percentage of hypochromic red blood cells, zinc protoporphyrin, and soluble transferrin receptors. Evidence to support these tests as targets for iron treatment is poor or unavailable.
Treat-to-target approaches. There are 2 widely used and effective approaches to IV iron treatment: (1) periodic iron repletion, consisting of a series of IV iron doses administered episodically to replenish iron stores whenever iron status tests decrease to less than target range; or (2) continuous maintenance treatment, consisting of smaller doses administered at regular intervals to maintain iron status tests stable within target. No RCTs are available to compare the efficacy and safety of these 2 approaches; however, the total cumulative dose may not differ between them.160 In studies that achieved steady-state ferritin levels and reported iron doses administered, the average IV iron dose needed to maintain a stable ferritin level (signifying achievement of the goal of neutral iron balance) appears to be in the range of 22 to 65 mg/wk (Table 28). Higher doses may lead to progressive increases in serum ferritin levels in some patients.148, 156
Iron Targets in Patients With ND-CKD and PD-CKD
Evidence to support an iron treatment target in patients with ND-CKD and PD-CKD is lacking. There are no RCTs comparing iron targets in these patient populations. Dialysis-related blood loss renders evidence from patients with HD-CKD unsuitable for application to patients with ND-CKD or PD-CKD. Thus, in these patients, the Work Group recommends iron treatment targets that reflect a conservative estimate of efficacy and a cautious approach to patient safety.
Upper Level of Ferritin
The statement There is insufficient evidence to recommend routine administration of IV iron if ferritin level if greater than 500 ng/mL reflects the findings of the Work Group that: (1) no RCTs have compared the safety and efficacy of ferritin targets greater than 500 ng/mL with the safety and efficacy of lower ferritin targets, (2) few studies have examined the efficacy of IV iron at ferritin levels greater than 500 ng/mL, (3) no study has examined either efficacy or safety beyond surrogate outcomes, (4) no information from interventional trials is available about the safety of ferritin targets greater than 500 ng/mL, and (5) sufficient evidence exists to suggest that tissue iron stores in patients with ferritin levels greater than 500 ng/mL are normal to greater than normal.
No evidence is available to compare ferritin targets greater than 500 ng/mL with lower ferritin targets. That is, no RCTs have assigned patients to a ferritin target greater than 500 ng/mL and compared results with those in patients assigned to a ferritin target less than 500 ng/mL. In the absence of comparisons between groups in intention to treat, no conclusions can be drawn to assess RRs and benefits of IV iron therapy at the higher ferritin level.
The efficacy of IV iron in patients with baseline ferritin levels greater than 500 ng/mL has been examined in a single uncontrolled study.150 Described previously, this trial administered IV iron to patients for 12 months, withheld iron for patients with a ferritin level greater than 1,000 ng/mL or TSAT greater than 50%, and found that mean ferritin level increased from 930 ng/mL at baseline to 1,383 ng/mL at 12 months and ESA dose decreased by 25%. However, the lack of a control group renders conclusions about the ESA dose change uncertain. Analysis of uncontrolled anemia interventional trials is subject to target bias. That is, patients who are most likely to survive and complete the trial likely bear a low disease burden and are, in turn, most likely to show higher Hb and lower ESA doses. Conversely, patients who are least likely to complete the trial bear a high disease burden and therefore are most likely to show worsening anemia and higher ESA doses. Successful trial completion therefore is associated with higher Hb levels and lower ESA doses, regardless of intervention.
Other efficacy trials have examined the functional relationship between baseline ferritin level and likelihood of a surrogate hematologic response to IV iron challenge (Table 24). However, none of these studies examined patients with baseline ferritin values greater than 500 ng/mL. Moreover, because efficacy of IV iron in the available interventional and functional iron studies is limited to surrogate outcomes (reticulocyte increase, Hb level increase, or ESA dose decrease),152-155, 161,162 evidence of direct patient benefit (including improved QOL, health, or survival) of IV iron administration in patients at ferritin values greater than 500 ng/mL is lacking.
Information on potential harm to patients similarly is lacking. Again, evidence to permit reliable assessment of risks and benefits can only come from RCTs assigning patients to intent-to-treat ferritin targets greater than 500 ng/mL compared with less than 500 ng/mL, with adequate power to assess both efficacy and safety.
Evidence that tissue iron stores in patients with ferritin levels greater than 500 ng/mL are adequate to greater than normal includes results from surveys in the general US population and studies of bone marrow iron or liver iron in patients with CKD. Among randomly selected individuals in the general US population, ferritin values greater than 500 ng/mL lie at or greater than the 95th percentile among males aged 15 to 59 years and far exceed the 95th percentile among females of all ages.163 However, findings in the general population do not necessarily apply to patients with CKD. Among patients with CKD undergoing bone marrow examination, no patient with a ferritin level greater than 500 ng/mL showed absent marrow iron stores.98,99 Finally, among patients with HD-CKD undergoing tissue iron determination by means of magnetic susceptometry, ferritin levels greater than 500 ng/mL were associated with hepatic non–heme-iron concentrations greater than the upper limit of normal.163 In short, ferritin values exceeding 500 ng/mL likely represent supraphysiological results in the general population and tissue iron sufficiency or excess in patients with HD-CKD.
Thus, no current evidence is available to support routine treatment of patients with serum ferritin levels greater than 500 ng/mL. Rather, clinicians should judge the individual patient's clinical status and ESA responsiveness and base iron treatment decisions on this assessment. In particular, when serum ferritin level is greater than 500 ng/mL while the concurrently measured TSAT is less than 20%, iron deficiency may be present and a course of iron treatment may be considered.
In short, no current evidence is available to support treating most patients with serum ferritin levels greater than 500 ng/mL. A therapeutic response to a 1,000-mg IV iron challenge in a patient with a ferritin level greater than 500 ng/mL is unlikely. Because iron stores are adequate or elevated in patients with ferritin values greater than 500 ng/mL, the long-term safety of iron therapy at target ferritin values exceeding 500 ng/mL is untested, and efficacy results of IV iron therapy are limited to surrogate outcomes rather than direct patient benefits, the Work Group favors limiting routine iron treatment to patients with serum ferritin values less than 500 ng/mL. Accordingly, the conclusion of the Work Group is that there is insufficient evidence to recommend routine administration of IV iron if ferritin level is greater than 500 ng/mL.
The finding of a TSAT less than 20% coupled with a ferritin level greater than 500 ng/mL poses a particularly difficult problem for clinicians.157 This situation may be caused by iron test variability,157 falsely low TSAT results, inflammation, or reticuloendothelial iron blockade. Evidence on the risks and benefits of IV iron therapy in these patients is almost entirely lacking. However, the statement that There is insufficient evidence to recommend routine administration of IV iron if ferritin level is greater than 500 ng/mL does not preclude IV iron administration in selected patients when, in the clinician's judgment, a trial of iron therapy is warranted.
It is important to reiterate that the statement There is insufficient evidence to recommend routine administration of IV iron if ferritin level is greater than 500 ng/mL refers to ferritin targets, not to achieved or acquired ferritin levels. Ferritin levels exceeding 500 ng/mL are often achieved as a result of iron therapy given to patients with lower baseline ferritin levels or may be acquired in the course of infection, inflammation, or other illness. Achieved or acquired ferritin levels are described elsewhere,164 but are not the subject of this guideline statement.165
Route of Administration
Evidence to support the statement The preferred route of iron administration is IV in patients with HD-CKD consists of results from 3 RCTs comparing IV iron with oral iron administration (Table 29A–Table 31A), including 2 that incorporated a placebo or nontreatment control arm (Table 30). A fourth RCT compared oral iron administration to no-iron control (Table 30). In 2 of these 4 RCTs, patients were assigned to IV or oral iron treatment while undergoing ESA therapy.159,166 By study completion, those assigned to IV iron showed a greater Hb level, lower ESA dose, or both compared with those assigned to oral iron (Table 29A, Table 31B). In the third RCT, patients not receiving ESA showed an increase in Hb levels from 7.8 to 11.0 g/dL after IV iron, but no change in Hb levels was seen in patients assigned to oral iron. Between-group comparisons were not reported.167 Of the 4 available RCTs, 3 included a placebo-treatment or no-iron-treatment arm. Results showed no difference in final Hb level or ESA dose between oral iron treatment and placebo/control. In summary, evidence in patients with HD-CKD supports 2 conclusions: (1) oral iron administration is not demonstrably more effective than either placebo or no treatment; and (2) IV iron administration is superior to oral iron administration.
A newer form of oral iron that has not been studied widely is heme iron polypeptide. A single RCT of patients with HD-CKD compared treatment with IV iron with treatment with heme iron polypeptide.168 Uncertainty in the randomization procedure, disproportionately high baseline ESA doses in the oral iron treatment group, a substantial dropout fraction confined to the oral iron treatment group, a significant decrease in serum ferritin levels during 6 months of oral iron treatment, and an undefined IV iron treatment protocol render the results of this trial difficult to interpret. Further studies will be required to provide a basis for recommendations on the use of this agent.
Evidence to support the statement The route of iron administration can be either IV or oral in patients with ND-CKD and PD-CKD consists of results from 4 RCTs comparing IV iron with oral iron administration in patients with ND-CKD (Table 29B, Table 31B). The Work Group rated the evidence from these trials as strong or moderately strong. There are no RCTs in patients with ND-CKD comparing oral iron therapy with placebo or no iron treatment. There are no RCTs in patients with PD-CKD.
The 4 available in ND-CKD RCTs differ substantially in the use, timing, and adjustment of ESA therapy (Table 31B). Three initiated ESA therapy in all study patients at randomization, whereas 1 continued ESA therapy in patients previously treated, but did not initiate ESA therapy in previously untreated patients. One adjusted ESA during the trial to achieve a target Hb level, whereas 3 permitted no ESA dose adjustment or required removal from the trial if ESA therapy initiation or ESA dose increase was needed.
The 4 available RCTs also differ in the dosing, frequency, and duration of IV iron administered. The 4 dosing regimens included 1,000 mg administered in 2 or 5 divided doses over 2 weeks,169 200 mg monthly,170 300 mg monthly,171 or 1,000 mg in 5 doses over 5 weeks.172
Finally, the 4 available RCTs differ substantially in severity of anemia at baseline, ranging from a mean Hb level of 5.8 to 6.3 g/dL in 1 trial170 to 9.7 to 10.2 g/dL in the remaining 3 trials.
Between-group differences showing superiority of IV iron over oral iron were seen in 2 of the 4 available RCTs (Table 31B). The 2 studies that initiated ESA therapy in patients with moderate anemia found no difference between patients assigned to IV iron compared with oral iron.171, 172 The 2 trials that showed differences either initiated ESA therapy simultaneously with iron therapy in patients with severe anemia170 or maintained prior ESA status without adjustment in patients with moderate baseline anemia.169 In the latter study, superiority of IV iron over oral iron was greatest among patients with ESA therapy and baseline Hb level less than 9.0 g/dL.169
Other outcomes compared in the 4 available RCTs included rate of decline in kidney function, QOL, adverse GI effects, dietary protein and energy intake, and adherence to prescribed therapy. Compared with patients assigned to oral iron therapy, those assigned to IV iron treatment showed no difference in rate of decrease in kidney function,169-171 no difference in QOL,169 fewer GI symptoms,169-172 no difference in protein and energy intake,171 and better adherence to prescribed therapy.169 Five patients in 2 trials, all women with low body mass, experienced adverse reactions to administration of IV iron, including hypotension, cramping, arthralgia, and myalgia.169,171
Given the variability in trial design, inconsistency of results, potential for adverse effects of IV iron, lack of information on potential adverse effects of oral iron, surrogate nature of tested outcomes in iron intervention trials, paucity of information on patient preference and adherence to treatment recommendations, and lack of information on the efficacy of oral iron compared with placebo or no-iron treatment, the Work Group concluded that the strength of available evidence is insufficient to support a guideline statement on the use of IV versus oral iron in patients with ND-CKD. However, in the opinion of the Work Group, the route of iron administration can be either IV or oral in patients with ND-CKD or PD-CKD.
Because no RCTs are available to compare IV iron with oral iron in patients with PD-CKD, the Work Group considered whether to regard the PD-CKD patient population as similar to ND-CKD, similar to HD-CKD, or too dissimilar from either to justify a recommendation. On the basis of the assumption that patients with PD-CKD do not experience ongoing dialysis-associated blood loss, the Work Group reasoned that patients with PD-CKD resemble the ND-CKD population and differ from patients with HD-CKD. Thus, patients with PD-CKD are included in the current opinion-based CPR.
Hypersensitivity Reactions
There currently are 3 forms of IV iron that are widely available: iron dextran, sodium ferric gluconate, and iron sucrose. All forms of IV iron may be associated with acute AEs, occasionally severe, comprised of hypotension with or without other symptoms and signs. The cause of the reactions is incompletely understood. Immune mechanisms (including mast cell–mediated processes leading to a clinical syndrome resembling anaphylaxis) may have a role in some cases. In others, the iron agent may release bioactive, partially unbound iron into the circulation, resulting in oxidative stress and hypotension (labile or free iron reactions). The pathogenesis may differ depending on the type of IV iron. Anaphylactoid reactions appear to occur more frequently with iron dextran,173 and labile or free iron reactions occur more frequently with nondextran forms of iron.174
The reported frequency of acute drug AEs depends in large part on the structure and rigor of the experimental design used. Direct patient observation after administration of an IV iron agent permits the most reliable assessment. For example, in a double-blind placebo-controlled trial of sodium ferric gluconate in 2,534 HD patients,175 investigators directly observed patients for evidence of reactions after blinded IV administration of drug or placebo. If, in the opinion of the investigator, a reaction was demonstrated, serum tryptase levels were measured to evaluate for mast cell–mediated hypersensitivity.
Prospective single-arm clinical trials also contribute information on drug-related AEs in the target population (Table 31). In reports of these trials, observation for drug-related AEs was performed by the study nurse or a staff nurse or, alternatively, the method of observation was not specified.
Retrospective study designs use review of medical records or large-scale electronic databases to identify potential drug-related AEs after the fact.176,177
Finally, pharmacovigilance surveillance studies use national voluntary reporting data sources, including the Freedom of Information Act database administered by the FDA, to evaluate AE profiles of IV iron agents. Pharmacovigilance studies yield unreliable information about absolute AE rates and are susceptible to uncontrolled observer bias, but afford the advantage of detecting rare events, shedding light on common characteristics of low-frequency events, and suggesting relative rates of AEs. However, they do not permit statistical comparison of AE rates for different IV iron agents.
The frequently nonspecific nature of IV iron–related AEs, the substantial overlap between drug-related AEs and dialysis-related AEs (eg, dizziness, dyspnea, cramps, pruritus, nausea, constipation, diarrhea, and hypotension), and the low anticipated event rate for the most serious AEs pose significant challenges for each study design described. The single greatest problem is the absence of generally accepted criteria to identify IV iron–related AEs and distinguish hypersensitivity from nonhypersensitivity reactions. The absence of criteria for IV iron–related AEs introduces potential observer bias into both prospective and retrospective trials. Pharmacovigilance studies also are subject to observer bias because only AEs thought to be drug related are reported. As long as neither descriptive criteria nor objective markers are available, designation of an AE as drug related likely will remain somewhat subjective. Although blinding the intervention so that neither the investigator nor the patient is aware of the identity of the agent administered improves the objectivity of AE reporting, event rates are sufficiently low to preclude comparative trials that are logistically feasible. For example, to conduct an RCT that would be adequately powered to compare serious AE rates between 2 iron agents would require enrolling more than 10,000 patients, an unlikely prospect. Accordingly, comparative assessment of safety is likely to continue to rely on retrospective chart review, analysis of large-scale medical databases, and pharmacovigilance reports.
Evidence is available from 2 retrospective reports, including 1 in patients with CKD. Both analyses concluded that the rate of life-threatening reactions to iron dextran administration is 0.6% to 0.7%.178,179 Two additional studies used large medical databases to further identify the nature and consequences of life-threatening reactions after IV iron dextran.
Fletes et al 2001.177 This study identified 165 suspected drug-related AEs after 841,252 exposures to iron dextran in patients with HD-CKD (rate per exposure, 0.02%). There was 1 fatal event. Although many AEs followed administration of a test dose or first dose, this study identified serious AEs in patients who had successfully received previous test or treatment doses. The majority of the non-naïve patients who experienced AEs after iron dextran administration did so at the time of the first dose of a planned series, suggesting that the risk for first-dose AE may recur in prevalent patients after an interval free from iron dextran exposure.177
Walters and Van Wyck 2005.176 The database of a large dialysis provider was examined to identify episodes of iron dextran–induced anaphylaxis sufficiently severe to require use of resuscitative medications. They found 7 events in a total of 48,509 patients treated. However, all 7 events occurred in previously unexposed patients after first or second exposure to the drug, yielding a slightly greater true incidence of 0.035%. The lower figure reported in this study compared with the 2 studies using chart review probably reflects the decision to study only the incidence of AE requiring in-center use of resuscitative medication.176 Of interest, the prevalence of suspected hypersensitivity in that study matched the findings of the previous retrospective reviews: 0.69% of prevalent patients were recorded as sensitive to iron dextran.
Pharmacovigilance surveillance studies yield information on deaths potentially related to IV iron. Deaths thought to be IV iron–related have been reported to the FDA for each of the IV iron agents available in the United States. In an analysis of US and European surveillance databases, 31 deaths occurring in association with 196 cases of iron dextran anaphylaxis were recorded in the United States between 1976 and 1996 compared with none with similar exposure rates with sodium ferric gluconate.180 Another group studied the FDA Freedom of Information surveillance database to compare severe AE rates for IV iron drugs.181 They confirmed higher anaphylaxis and fatality event rates for iron dextran than for the nondextran irons. These results should be interpreted with caution given that AEs for drugs in the marketing phase probably are grossly underreported for all agents; definitions for AEs are not generally accepted, as we have noted; and the primary intention of the database is to generate signals of unexpected AEs, not to compare drugs.182 Nonetheless, these results add to the body of evidence indicating a trend toward more frequent and more severe reactions with iron dextran.
Taking the pharmacovigilance information together with evidence that iron dextran AEs may occur in patients who have successfully received a previous test dose or series of therapeutic doses, we recommend that resuscitative medication and personnel trained to evaluate and resuscitate anaphylaxis accompany each administration of iron dextran.
Targets of Iron Therapy
There are a number of serious limitations to the evidence supporting iron status targets. ESA dose and Hb level response as surrogates for iron efficacy are universal among iron intervention trials (IV iron challenge studies), RCTs, and uncontrolled prospective trials. No iron treatment trials have been designed or are sufficiently powered to yield information on outcomes, including the crucial issue of safety, that are directly important to patients. Of the 2 available RCTs comparing iron status targets, 1 used TSAT targets,108 1 used ferritin targets,149 both were small, neither examined safety, and the TSAT trial showed increasing non–steady-state ferritin levels in the higher TSAT target arm. Conversely, IV iron challenge studies yield information that is limited to the acute hematologic response to a dose of IV iron—results that bear little relevance to optimum treatment targets for ongoing iron administration. Moreover, even when limited to predicting the acute response to IV iron challenge, available iron status tests perform poorly, yielding relatively low sensitivity and specificity over a range of cutoff values, flat receiver-operator characteristic curves, and low area under the curve. Finally, although stainable bone marrow iron may yield important information on the likelihood of storage iron depletion or iron excess or on the relationship between iron stores and results of serum iron status tests, the relevance of this information to setting optimum treatment targets for iron status is uncertain. In short, iron status targets as goals for treatment require treat-to-target RCTs to provide comparative evidence of efficacy and safety. However, available evidence is of limited quality or is altogether lacking.
Upper Level of Ferritin
The limitations of available evidence, described previously, provide the rationale for the cautious use of IV iron at high ferritin levels.
Route of Administration
In patients with HD-CKD, available RCTs are small, thereby limiting their potential applicability to unselected HD-CKD target populations. As in all IV iron studies to date, outcomes are limited to the surrogate Hb levels and ESA doses. Although these outcomes have financial implications for the total cost of anemia management, the comparative cost of anemia management using IV versus oral iron treatment has not been examined, and the relationship, if any, between surrogate outcomes and outcomes that are important to patients has not been determined. Moreover, although managing anemia requires chronic and ongoing care, most clinical trials involving therapeutic iron intervention are of relatively short duration; thus, the long-term effects of iron therapy remain unknown. Resolution of these issues, particularly in the ND-CKD and PD-CKD patient population, clearly is needed before a conclusive assessment of net benefit can be made.
Other Limitations
AEs thought to be related to labile iron require a decrease in the dose or rate of infusion or both. AEs thought to be related to hypersensitivity to the agent require stopping the agent and preclude further administration.