ESAs are critical components in managing the anemia of CKD. Available ESAs are each effective in achieving and maintaining target Hb levels. Aspects of administration may differ between short-acting and long-acting agents.
3.1.1 Frequency of Hb monitoring:
3.1.1.1 In the opinion of the Work Group, the frequency of Hb monitoring in patients treated with ESAs should be at least monthly.
3.1.2 ESA dosing:
3.1.2.1 In the opinion of the Work Group, the initial ESA dose and ESA dose adjustments should be determined by the patient's Hb level, the target Hb level, the observed rate of increase in Hb level, and clinical circumstances.
3.1.2.2 In the opinion of the Work Group, ESA doses should be decreased, but not necessarily withheld, when a downward adjustment of Hb level is needed.
3.1.2.3 In the opinion of the Work Group, scheduled ESA doses that have been missed should be replaced at the earliest possible opportunity.
3.1.2.4 In the opinion of the Work Group, ESA administration in ESA-dependent patients should continue during hospitalization.
3.1.2.5 In the opinion of the Work Group, hypertension, vascular access occlusion, inadequate dialysis, history of seizures, or compromised nutritional status are not contraindications to ESA therapy.3.1.3 Route of administration:
3.1.3.1 In the opinion of the Work Group, the route of ESA administration should be determined by the CKD stage, treatment setting, efficacy, safety, and class of ESA used.
3.1.3.2 In the opinion of the Work Group, convenience favors subcutaneous (SC) administration in non–HD-CKD patients.
3.1.3.3 In the opinion of the Work Group, convenience favors intravenous (IV) administration in HD-CKD patients.3.1.4 Frequency of administration:
3.1.4.1 In the opinion of the Work Group, frequency of administration should be determined by the CKD stage, treatment setting, efficacy considerations, and class of ESA.
3.1.4.2 In the opinion of the Work Group, convenience favors less frequent administration, particularly in non–HD-CKD patients.
The decision to start ESA therapy in a patient with CKD-associated anemia invokes an additional series of decisions that must be made before the first dose is administered. Each of the following issues must be considered before moving forward: choosing an ESA, an initial ESA dose, and the route and frequency of ESA administration; planning a Hb level monitoring schedule; predicting a desired rate of increase in Hb level; and anticipating ESA dose adjustments for Hb results, AEs, or intercurrent hospitalizations. Making informed decisions requires understanding the method of action of ESAs, then selecting those which best fit the patient, the patient-care setting, and the constraints of the health care delivery system from the range of choices that are known to be safe and effective.
Definitions
The term ESA applies to all agents that augment erythropoiesis through direct or indirect action on the erythropoietin receptor. Currently available ESAs include epoetin alfa, epoetin beta, and darbepoetin. Epoetin alfa and beta have been designed to resemble closely the endogenous molecule and have similar pharmacokinetics. They are considered “short-acting” in comparison to darbepoetin, a second-generation molecule with a prolonged half-life, which is considered “long-acting.”
Frequency of Hb Monitoring
The statement The frequency of Hb monitoring in patients treated with ESA should be at least monthly is intended to provide sufficient surveillance information to assist in achieving and maintaining target Hb levels safely and expeditiously during ESA therapy. This recommendation, which fits common practice, follows a chain of reasoning. The minimum interval between ESA dose adjustments is 2 weeks because the effect of most dose changes will not be seen within a shorter interval. Consideration of an ESA dose adjustment is based on the next projected Hb level. Because the accuracy of projection (extrapolation) increases with the number of contributing data points, the frequency of Hb monitoring is likely to be an important determinant of the accuracy of ESA dose adjustment. Evidence to support this line of reasoning is indirect. Several RCTs have randomized HD patients with target-range Hb levels to a change in frequency of ESA administration, a change in ESA class, or both. RCTs that have monitored Hb values weekly and adjusted ESA doses as frequently as every 2 weeks have achieved stable Hb levels early after randomization.136-138 A single RCT that monitored Hb levels and considered ESA dose adjustment monthly required 6 to 9 months to stabilize Hb levels after randomization,139 but mean Hb level remained within the target range for that trial.
Within the recommended ranges for monitoring and dose adjustment, unstable Hb level, out-of-target Hb level, and HD favor shorter intervals, whereas stable Hb level, within-target Hb level, PD, ND-CKD, and minimizing laboratory resource utilization favor longer intervals. The frequency of ESA dose adjustment is unaffected by length of action: during an 8-week period, given Hb monitoring weekly, short-acting ESA thrice weekly or long-acting ESA once weekly, and consideration for dose adjustment every 2 weeks, 44% to 49% of patients will actually require dose adjustment.138
ESA Dosing
The initial ESA dose and ESA dose adjustments should be determined by the patient's Hb level, the target Hb level, the observed rate of increase in Hb level, and clinical circumstances.
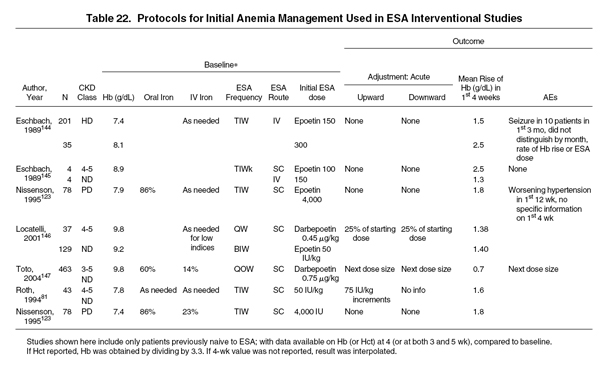
This statement aims to guide medical decision making so that the initial rate of increase in Hb levels is in keeping with the patient's current status, the treatment setting, and the patient's need for anemia treatment. In general, the objective of initial ESA therapy is a rate of increase in Hb levels of 1 to 2 g/dL per month. Evidence for the safety of initial ESA therapy derives principally from interventional trials in patients not previously treated with ESAs. In patients with CKD-associated anemia and initial Hb levels less than target range, these trials have shown the mean initial rate of Hb level increase to be in the range of 0.7 to 2.5 g/dL in the first 4 weeks. The rate of increase varies greatly and depends in part on the patient population, initial dose, dosing frequency, and route of administration (Table 22). Hypertension and seizures were noted in the first 3 months after initiating therapy in severely anemic patients, but it is unclear whether these events occurred within the first 4 weeks and whether they were related to the rate of increase in Hb levels.
In general, ESA dose adjustments were not made in the first 4 weeks of trials for which results are available. The frequency of ESA dose adjustment thereafter should be determined by the rate of increase in Hb levels during initial ESA therapy, the stability of Hb levels during maintenance ESA therapy, and the frequency of Hb testing. The minimum interval between ESA dose adjustments in the outpatient setting generally is 2 weeks because the effect of most dose changes will not be seen within a shorter interval.
ESA doses should be decreased, but not necessarily held, when a downward adjustment of Hb level is needed. Withholding ESA doses, particularly for long periods, may lead to a delayed decrease in Hb levels to less than target range. Such a decrease may initiate periodic cycling of Hb levels at greater than and less than the target Hb range.114 This finding is in keeping with the mechanism of action of ESA in preventing apoptotic death of CFU-Es and early erythroblasts. Should ESA doses be withheld in an ESA-dependent patient, a prolonged loss of erythropoietic precursors may result. Accordingly, the Work Group recommends that ESA doses not be withheld routinely for Hb levels greater than target range, hospitalization, poorly controlled hypertension, or vascular access occlusion.
The statement Scheduled ESA doses that have been missed should be replaced at the earliest possible opportunity reflects the concern that missed doses of ESA, like held doses, may lead to a delayed decrease in Hb levels to less than target range. This is illustrated most clearly in patients who receive ESA dosing every 2 to 4 weeks, but the principle applies to all patients undergoing ESA therapy.
Similarly, the statement Hypertension, vascular access occlusion, inadequate dialysis, history of seizures, or compromised nutritional status are not contraindications to ESA therapy aims to discourage the practice of withholding ESA therapy in the presence of conditions potentially linked to anemia treatment. If these disorders arise in the course of anemia therapy with ESA, they should be treated appropriately with specific measures. There is no evidence that withholding ESA therapy improves patient outcomes in these settings.
Route of Administration
The route of administration should be determined by the CKD stage, treatment setting, efficacy considerations, and the class of ESA used. Among patients with HD-CKD, either SC or IV administration is possible. In the outpatient setting, SC administration is the only routinely feasible route of administration for patients with PD-CKD and ND-CKD. Among patients with HD-CKD, either SC or IV administration is feasible, but the risk for pure red cell aplasia (PRCA) associated with SC administration, albeit small, recently prompted the US Food and Drug Administration (FDA) to recommend the IV route. The relationship between SC ESA administration and risk for PRCA experienced outside the United States is discussed elsewhere (Guideline 3.5).
Among short-acting ESAs, efficacy of SC administration in patients with HD-CKD is superior to that of IV administration. Although many trials have achieved similar results, the findings of a single large multicenter RCT are sufficient to establish the strength of evidence for this guideline statement.140 In this trial, patients with HD-CKD were eligible for randomization only if they were currently receiving epoetin alfa and their Hct was within the target range. Upon randomization (from SC or IV to IV or SC), ESA doses were first decreased to allow Hb levels to decrease to less than target range. Doses then were titrated upward to again achieve target Hct levels, then were adjusted to maintain Hct in the target range during a 26-week maintenance phase. Among patients who completed the trial, those assigned to SC administration showed 27% lower ESA doses than those assigned to IV administration. Mean achieved Hcts did not differ between groups. Of the patients who, by assignment, switched from IV to SC administration, 58% showed a decrease in ESA dose and 23% showed an increase; among their counterparts who switched from SC to IV administration, the corresponding proportions were 28% and 49%, respectively. In short, SC administration of short-acting ESAs is associated with an approximately 30% decrease in ESA dose for the same target Hb outcome. However, not all patients show a dose decrease after conversion from IV to SC, and some will show a dose increase.
Among long-acting agents, efficacy of SC compared with IV administration appears to be equivalent at examined dosing frequencies.137,141
Frequency of Administration
Frequency of administration should be determined by the CKD treatment setting and the class of ESA. Selecting frequency of ESA administration may require a choice between maximum efficacy and optimum convenience and comfort. Maximum efficacy occurs within dosing intervals that are ESA class specific. For example, in patients with HD-CKD receiving SC short-acting ESA therapy, ESA efficacy decreases when the dosing is extended from thrice-weekly to once-weekly administration.139 When every-2-week administration of long-acting ESAs is extended to every 4 weeks, efficacy either remains stable142 or decreases incrementally.143
In practice, the use of ESAs is subject to wide variability that reflects broad differences in practice setting, patient preference, and reimbursement constraints. The definition of best practice therefore should reflect both available evidence and prevailing conditions, preferences, and priorities. The evidence base for CPRs on the use of ESAs requires information generated from quality improvement programs and anemia management protocols that examine multiple interventions and explicitly state underlying goals and assumptions.