NKF KDOQI GUIDELINES
KDOQI Clinical Practice Guidelines and Clinical Practice Recommendations for Anemia in Chronic Kidney Disease
II. CLINICAL PRACTICE GUIDELINES AND CLINICAL PRACTICE RECOMMENDATIONS FOR ANEMIA IN CHRONIC KIDNEY DISEASE IN ADULTS
CPR 3.5. EVALUATING AND CORRECTING PERSISTENT FAILURE TO REACH OR MAINTAIN INTENDED HB
Although relative resistance to the effect of ESAs is a common problem in managing the anemia of patients with CKD and is the subject of intense interest, the bulk of available information suggests that—in the absence of iron deficiency—there are few readily reversible factors that contribute to ESA hyporesponsiveness.
3.5.1 Hyporesponse to ESA and iron therapy:
In the opinion of the Work Group, the patient with anemia and CKD should undergo evaluation for specific causes of hyporesponse whenever the Hb level is inappropriately low for the ESA dose administered. Such conditions include, but are not limited to:
- A significant increase in the ESA dose requirement to maintain a certain Hb level or a significant decrease in Hb level at a constant ESA dose.
- A failure to increase the Hb level to greater than 11 g/dL despite an ESA dose equivalent to epoetin greater than 500 IU/kg/wk.
3.5.2 Evaluation for PRCA:
In the opinion of the Work Group, evaluation for antibody-mediated PRCA should be undertaken when a patient receiving ESA therapy for more than 4 weeks develops each of the following:
- Sudden rapid decrease in Hb level at the rate of 0.5 to 1.0 g/dL/wk, or requirement of red blood cell transfusions at the rate of approximately 1 to 2 per week, AND
- Normal platelet and white blood cell counts, AND
- Absolute reticulocyte count less than 10,000/µL.
BACKGROUND
Hyporesponsiveness to ESAs
Hyporesponsiveness to ESAs is a common finding of grave significance whether it is manifested by persistent, below-target Hb levels despite substantial ESA doses or by within-target Hb levels attained only at very high ESA doses. In patients with HD-CKD undergoing ESA therapy, Hb levels less than 11 g/dL are associated with increased mortality and hospitalization rates, and failure to achieve an Hb level greater than 11 g/dL is a poor prognostic sign. Given the disproportionate burden of morbidity and mortality that the hyporesponsive patient population bears and the ESA expense that hyporesponsiveness engenders, hyporesponsiveness to ESAs deserves more scrutiny than it has received. Although most disorders associated with hyporesponsiveness are readily apparent, a review of available information on patients with coexisting hematologic or oncological disorders may be worthwhile. Similarly, a rare disorder, PRCA, deserves special consideration.
Antibody-Mediated PRCA
Rarely, patients undergoing ESA therapy develop antibodies that neutralize both ESA and endogenous erythropoietin. The resulting syndrome, antibody-mediated PRCA, is characterized by the sudden development of severe transfusion-dependent anemia. Rapid recognition, appropriate evaluation, and prompt intervention can be effective in limiting the consequences of this life-threatening hyporesponse condition.
Antibody-mediated PRCA, although rare in patients administered ESAs, received urgent attention after 1998. Between 1989 and 1998, three reports described the development of PRCA in a small number of patients with CKD administered ESAs.273,274 Reports of PRCA increased sharply in 1998 and reached a peak in 2002 (Fig 17). 273,275 These reports were associated with SC administration of an epoetin alfa formulation not available in the United States. In 1998, in this formulation, polysorbate 80 was used to replace human albumin. Preparations of this product included single-dose syringes fitted with uncoated rubber stoppers and prefilled with epoetin alfa. Results of an intensive investigation indicate that in the presence of polysorbate 80 and uncoated rubber can release organic compounds that may act as immunoadjuvants, thereby increasing the immunogenicity of SC-administered epoetin alfa.273,275
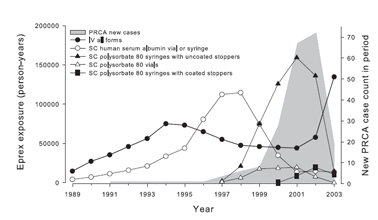
Fig 17. Exposure to Eprex® and case counts of PRCA. Relationship between reporting rate of new cases of PRCA and route of administration (SC versus IV), stabilizer (human serum albumin versus polysorbate 80), and coated versus uncoated stoppers in preparations of Eprex®, a form of epoetin alfa marketed outside the United States. Reprinted with permission.275
Between 2001 and late 2003, single-dose syringes with polysorbate 80 and uncoated stoppers were replaced by syringes with fluoro-resin-coated stoppers.275 In addition, SC administration of the agent had been prohibited in Europe and discouraged in Canada. By 2004, the incidence of new antibody-mediated PRCA had decreased to pre-1998 levels.
Isolated cases of PRCA have been observed in association with the use of other ESAs.273,274,276-,279 No case of antibody-associated PRCA has been documented in patients treated with only IV administration of ESAs.274 An increase in antibody-mediated PRCA has not been seen among patients in the United States, where the immunogenic formulation has not been available.
The incidence rate of PRCA in patients who were exposed to SC-administered ESA from syringes with uncoated stoppers and polysorbate 80 is estimated at 4.23 cases/10,000 patient-years.275 The incidence with SC use of all other forms of SC-administered ESA is estimated to be 0.5 cases/10,000 patient-years.275 The finding that antibody-mediated PRCA develops only rarely, even among patients exposed to adversely equipped syringes, suggests that additional factors must have been involved to render ESA immunogenic, potentially including differences in host immunoreactivity or product storage and handling.
RATIONALE
Hyporesponse to ESA and Iron Therapy
The patient with anemia and CKD should undergo evaluation for specific causes of hyporesponse if Hb level is persistently less than 11 g/dL AND if ESA doses are equivalent to epoetin greater than 500 IU/kg/wk. Results from the USRDS national data system show that the distribution of epoetin alfa doses is quite broad, the number of administrations per percentile range is relatively constant over the full spectrum of doses, and the relationship between percentile range and mean epoetin dose per administration is distinctly nonlinear (Fig 18). In these unselected patients, the 99th percentile doses are 30 times greater than the 1st percentile doses, and the top 20% of patients seem to be using a disproportionate amount of ESA compared with the lower 80%.
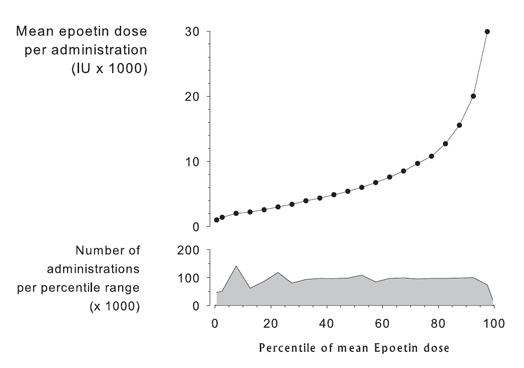
Fig 18. Mean epoetin dose per patient per administration by percentile of dose (1st, 5th to 95th, and 99th). Data are for December 2004, courtesy of USRDS.
Available information on hyporesponsiveness is weakened by the lack of a validated quantitative measure of ESA resistance. Although a resistance index—calculated by dividing the weight-adjusted ESA dose by Hb level—has been proposed, precisely how the ESA dose and Hb level should be determined (area under the curve, single value, time averaged) has not been standardized and reference values have not been validated.
Factors most commonly associated with persistent failure to achieve target Hb levels for at least 6 months despite ESA therapy include the following280:
- Persistent iron deficiency
- Frequent hospitalization
- Hospitalization for infection
- Temporary catheter insertion
- Permanent catheter insertion
- Hypoalbuminemia
- Elevated C-reactive protein level.
In general, these problems do not pose a diagnostic challenge or yield to simple solutions. A second set of disorders also may be identified among hyporesponsive patients. Unfortunately, they also are found among those who do not meet criteria for hyporesponsiveness, and they also represent neither frequent nor elusive diagnoses280:
- Pancytopenia/aplastic anemia
- Hemolytic anemia
- Chronic blood loss
- Cancer, chemotherapy, or radiotherapy
- Inflammatory disease
- Acquired immune deficiency syndrome
- Infection.
Only a small percentage of patients with a Hb level less than 11 g/dL fail to respond to ESA therapy. Persistency analysis shows that approximately 10% of patients with HD-CKD who enter a 6-month period with a Hb level less than 11 g/dL remain at less than that threshold for 6 consecutive months.112 Among patients with a Hb level less than 11 g/dL administered high ESA doses (epoetin alfa > 30,000 IU/wk), only 0.6% of patients remained at less than that threshold for 6 consecutive months.
In short, the available evidence suggests that approximately 20% of patients with HD-CKD in the United States are administered ESA doses in excess of an epoetin equivalent of 30,000 IU/wk, or 428 IU/kg/wk for a 70-kg patient (Fig 18). Approximately 10% of patients with an Hb level less than 11 g/dL will persistently fail to attain a target Hb level of 11 g/dL or greater. However, among US patients with an Hb level less than 11 g/dL and ESA doses in excess of 30,000 IU/wk epoetin equivalents, less than 1% will remain at less than the Hb target for 6 months.
The patient with anemia, CKD, and a preexisting hematologic disorder represents an uncommon, but challenging, cause of ESA hyporesponsiveness and deserves special consideration. The quality of reviewed material is insufficient to provide specific recommendations. However, a brief review of the available literature may prove helpful to medical decision making in the treatment of these patients.
Management of anemia in patients with CKD with preexisting hematologic disorders associated with anemia may present specific problems because of multifactorial causes. In some patients, anemia may result predominantly from low endogenous erythropoietin levels and can be corrected readily by administration of ESAs. In other disorders, impaired marrow function, ineffective erythropoiesis, and shortened red blood cell survival may contribute to anemia and ESA hyporesponsiveness.
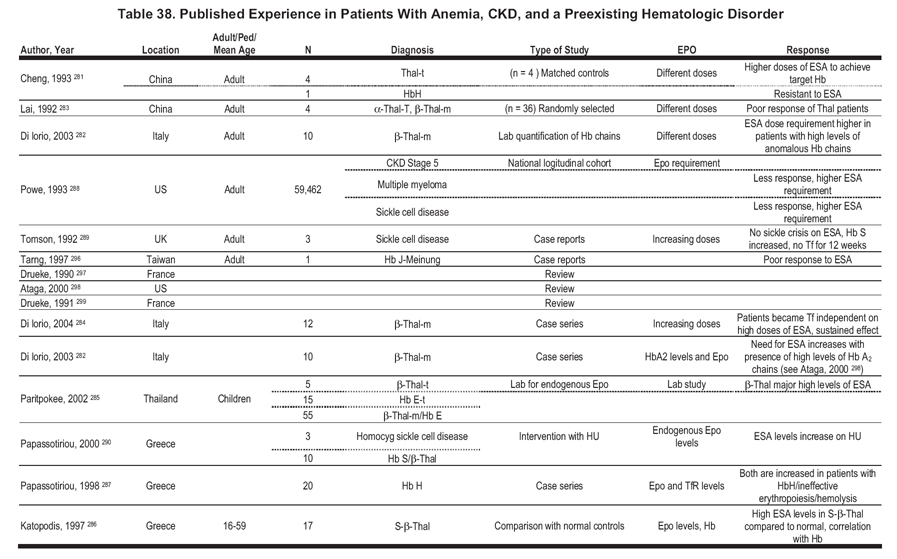
We identified a small number of publications that evaluated ESA responsiveness in patients with CKD and preexisting hematologic disorders (Table 38). The publications are predominantly observational, taking the form of individual case reports or reports of a small series of patients. Although the available information is insufficient to support guidelines or CPRs, the following summary statements may be helpful:
Thalassemia
- Patients with thalassemia have a poor response to ESA.281-283
- Higher doses of ESA are required to achieve target Hb levels.281-284
- Patients can become independent of transfusions on high doses of ESA.284
- The need for ESA increases in patients with high levels of HbA2 chains in the serum.282
- Patients with β-thalassemia major show high endogenous erythropoietin levels.285
- Patients with HbS/β-thalassemia have a higher-than-normal endogenous erythropoietin level.286
Hb H
- Patients are resistant to ESA.281, 287
- Endogenous serum erythropoietin levels and serum transferrin receptor levels are both increased.287
- The mechanism of anemia is likely related to ineffective erythropoiesis and hemolysis.287
Hb S
- Patients show higher ESA dose requirement to achieve target Hb level.288,289
- There may be a lower incidence of sickle cell crises while on ESA therapy.289
- Endogenous erythropoietin levels increase with hydroxyurea therapy.290
Multiple Myeloma
- Higher levels of ESA are required to achieve target Hb levels in patients with HD-CKD.288
- Therapy with ESAs reduces transfusions and improves QOL in anemic patients with or without kidney disease.291
General Comments
Patients with β-thalassemia and Hb S disease may require higher doses of ESA compared with patients with CKD who do not have hematologic disorders. There is no apparent contraindication to increase the ESA dose in either disorder. Some patients may require doses usually used in hematopoietic disorders (40,000 to 60,000 U/wk).
Evaluation for PRCA
Evaluation for antibody-mediated PRCA should be undertaken when a patient receiving ESA therapy for more than 4 weeks develops each of the following: sudden rapid decline in Hb level at the rate of 0.5 to 1.0 g/dL/wk, or requirement of red blood cell transfusions at the rate of approximately 1 to 2 per week; normal platelet and white blood cell counts; and absolute reticulocyte count less than 10,000/µL.
Syndrome Recognition
The characteristic sign of PRCA is almost complete cessation of erythropoiesis. Accordingly, a patient affected with PRCA evidences a decrease in Hb level of about 0.1 g/dL per day and a reticulocyte count less than 10,000/µL, consistent with the normal rate of red blood cell destruction and an absence of red blood cell production. Because nonerythroid marrow is unaffected, leukocyte and platelet counts are expected to be normal.274,276
Diagnostic Evaluation
Recommendations based on expert opinions have been published to guide the workup and therapy of patients suspected to have antibody-mediated PRCA.274,277,279 The definitive diagnosis is dependent upon demonstration of the presence of neutralizing antibodies against erythropoietin.
Management and Treatment
It is prudent to discontinue the administration of any ESA product in patients with suspected and confirmed diagnosis because the antibodies are cross-reactive and continued exposure may lead to anaphylactic reactions.292 Patients likely will require transfusion support. Treatment with immunosuppressive approaches is effective in a significant number of patients.293 Renal allografts usually result in a rapid decrease in antibody titers associated with therapeutic benefit.293 It is not clear whether treatment with ESA can be resumed safely after clearance of the antibody. Based on very preliminary data, this might be feasible; however, caution is advised.294 A single case also has been reported in which Hb levels could be restored in the presence of antierythropoietin antibodies by switching to a different product.295


