In CKD Patients (Stages 3 and 4):
5.1 If phosphorus or intact PTH levels cannot be controlled within the target range (see Guidelines 1, 3), despite dietary phosphorus restriction (See Guideline 4), phosphate binders should be prescribed. (OPINION)
5.2 Calcium-based phosphate binders are effective in lowering serum phosphorus levels (EVIDENCE) and may be used as the initial binder therapy. (OPINION)
In CKD Patients With Kidney Failure (Stage 5):
5.3 Both calcium-based phosphate binders and other noncalcium-, nonaluminum-, nonmagnesium-containing phosphate-binding agents (such as sevelamer HCl) are effective in lowering serum phosphorus levels (EVIDENCE) and either may be used as the primary therapy. (OPINION)
5.4 In dialysis patients who remain hyperphosphatemic (serum phosphorus >5.5 mg/dL [1.78 mmol/L]) despite the use of either of calcium-based phosphate binders or other noncalcium-, nonaluminum-, nonmagnesium-containing phosphate-binding agents, a combination of both should be used. (OPINION)
5.5 The total dose of elemental calcium provided by the calcium-based phosphate binders should not exceed 1,500 mg/day (OPINION), and the total intake of elemental calcium (including dietary calcium) should not exceed 2,000 mg/day. (OPINION)
5.6 Calcium-based phosphate binders should not be used in dialysis patients who are hypercalcemic (corrected serum calcium of >10.2 mg/dL [2.54 mmol/L]), or whose plasma PTH levels are <150 pg/mL (16.5 pmol/L) on 2 consecutive measurements. (EVIDENCE)
5.7 Noncalcium-containing phosphate binders are preferred in dialysis patients with severe vascular and/or other soft tissue calcifications. (OPINION)
5.8 In patients with serum phosphorus levels >7.0 mg/dL (2.26 mmol/L), aluminum-based phosphate binders may be used as a short-term therapy (4 weeks), and for one course only, to be replaced thereafter by other phosphate binders. (OPINION) In such patients, more frequent dialysis should also be considered. (EVIDENCE)
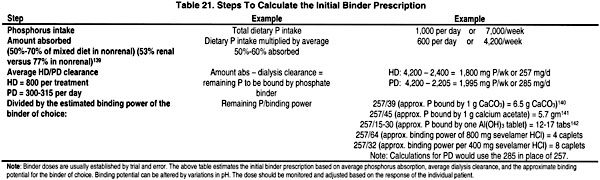
When dietary phosphate restriction is inadequate to control serum levels of phosphorus and/or PTH, the second line of therapy is the administration of phosphate binders. Different phosphate binder compounds have been utilized to control serum phosphorus levels, but the search still continues for the best possible binder. It is generally accepted that no one binder is effective and acceptable to every patient. Most commonly, a combination of binders may be used to control serum phosphorus levels to minimize the potentially serious side effects of any specific binder. The willingness of the patient to adhere to the binder prescription is paramount to control phosphorus absorption from the gastrointestinal tract and subsequently serum phosphorus level. Table 21 describes the steps to calculate the initial prescription of phosphate binders, and Table 22 provides the characteristics of various phosphate-binding agents.
The goal of phosphate-binder therapy is to maintain serum phosphorus levels within the range as outlined in Guideline 3 without negatively impacting nutritional status or causing serious side-effects. Thus, it is logical to initiate phosphate binder therapy when:
(1) Serum phosphorus levels are elevated, even though the patient is compliant with a dietary phosphate restriction;
(2) The serum phosphorus levels can be controlled by a dietary phosphate restriction only, but such dietary intervention hinders the intake of other critical nutrients;
(3) Blood PTH levels remain elevated after dietary phosphate restriction, even if the serum phosphorus levels are not elevated.
During the use of aluminum-based phosphate binders, patients should be monitored to avoid additional morbidity described with prolonged use of aluminum-containing phosphate binders146,147 to avoid aluminum toxicity (see Guidelines 11 and 12). The majority of research in the recent decade has focused on calcium-based binders, but other binder forms are now available. With recent concern about soft-tissue calcification which may be worsened by calcium-based phosphate binders, these noncalcium, nonaluminum binders are being used more frequently.
Increasing frequency of dialysis can enhance phosphorus clearance in hemodialysis patients. Among patients treated with thrice-weekly nocturnal hemodialysis in Tassin, France, serum levels of phosphorus were reduced despite increased dietary intake and reduced use of binders.148 Some patients treated with nocturnal dialysis six times per week have required phosphate supplements in the dialysate.149 Where the escalation of phosphate-binder dose is incapable of controlling serum phosphorus levels or not tolerated, increasing dialysis time, and—if possible—frequency (4 or more times per week) should be strongly considered.
In order to determine what the best phosphate binder is, studies that evaluated the efficacy and adverse effects of phosphate binders were analyzed. There were no prospective, controlled studies that evaluated phosphate binders in CKD Stages 3 and 4. However, since serum PTH levels in these patients are elevated due to phosphate retention, it was the opinion of the Work Group that the use of phosphate binders may become necessary if the serum levels of intact PTH could not be lowered to the target levels (see Table 15, Guideline 1) by dietary phosphate restriction and/or vitamin D therapy.
In CKD Stage 5, there were 16 prospective, controlled studies that evaluated 552 patients for various outcomes to quantify the efficacy of serum phosphorus control by phosphate binders. In all these studies, the patients were treated with dialysis and the primary focus of the analysis was on the use of calcium carbonate and calcium acetate, although some data on aluminum hydroxide, calcium gluconate, calcium carbonate plus magnesium carbonate, and sevelamer HCl were also available. It was possible to use these studies to perform a meta-analysis to compare the efficacy of the phosphate binders on outcomes, including: serum levels of phosphorus, PTH and calcium; bone biochemical markers; and extraskeletal calcification.143,150,151 No studies evaluated the effect of phosphate binders on patient quality of life, mortality rate, incidence of bone disease or fractures, or bone histomorphometry by bone biopsy measurements.
Fig 10. Meta-analysis of size of effect on serum phosphorus levels of calcium acetate versus calcium carbonate.
Two studies included a placebo group for comparison against calcium acetate143 and sevelamer,157 and both showed a superior efficacy of these binders compared to placebo.
There was only a single study evaluating magnesium as a phosphate binder: it is a crossover study that evaluated patients on calcium carbonate compared to a combination of calcium carbonate and magnesium carbonate.158 The magnesium arm had equivalent phosphorus control. However, the Work Group cautions that, in this study, the magnesium concentration in the dialysate was decreased. This is difficult to do in most units due to centralized dialysate delivery systems. Furthermore, there are no long-term studies on the safety and efficacy of magnesium as a phosphate binder, and thus the Work Group agreed that the use of magnesium-based phosphate binders may be justified only if all other compounds fail and the appropriate precautions are undertaken.
Effect on Calcium and Calcium-Phosphorus Product. Ten studies evaluated the effect of different phosphate binders on corrected serum calcium levels, ionized calcium, total calcium, or calcium-phosphorus product.143,153-157,159-162 Five of these studies compared different binders to calcium carbonate,143,154,155,158,161 but a meta-analysis failed to detect a difference in the corrected serum calcium levels. A placebo-controlled study found higher total calcium levels and lower calcium-phosphorus product in the calcium acetate-treated group compared to placebo.143 Although the overall change in serum calcium levels in 10 studies was not affected, meta-analysis of the data showed that calcium carbonate led to more hypercalcemic events compared to other phosphate binders, or when directly compared to calcium acetate only (Fig 11).151,153-156,158,160-163
Fig 11. Meta-analysis of size of hypercalcemic effect of calcium carbonate versus other phosphate binders.
Six studies assessed calcium-phosphorus product, 1 placebo-controlled and the others comparing different phosphate binders. Differences were observed in only 2 of these studies: calcium acetate led to a lower calcium-phosphorus product than placebo,143 and calcium carbonate led to a greater product than calcium ketoglutarate.162 This latter study found that ionized calcium levels were higher in patients treated with calcium carbonate compared to calcium ketoglutarate.162 Thus, the available data do not provide guidance regarding the choice of the appropriate calcium-based phosphate binder. The choice is a prerogative of the physician and depends on the binder’s tolerance by the patient.
Other Outcomes. The major side-effects observed as a result of phosphate-binder therapy were hypercalcemia, as described above, or gastrointestinal side effects. A meta-analysis indicated that gastrointestinal side effects were lowest with patients treated with calcium carbonate compared to other binders, although the effect size was small and thus no firm conclusions could be reached. 154,156,158,160,162,163
Six studies evaluated the effect of phosphate binders on nutritional outcomes,150,152,157-159,162 but different outcome measures were utilized, precluding comparative analyses. Two studies found that sevelamer led to lower serum cholesterol levels compared to placebo or calcium acetate, primarily due to a decrease in LDL cholesterol levels. 152,158
Patient compliance with prescribed binder therapy was not consistently reported, but ranged from 30% to 100%.132,138,164-170 None of the available data dealt with the effect of noncompliance on clinical outcomes. One study suggested that noncompliance was related to gastrointestinal side effects. While maintenance of high serum phosphorus levels could be due to noncompliance with phosphate binders, other factors, such as dietary indiscretion and phosphate release from the bone must also be considered.
There are few studies that demonstrated optimal timing for ingestion of phosphate binders, but the general consensus among the Work Group is that binders should be taken 10 to 15 minutes before, or during, the meal.
In comparing calcium carbonate and aluminum hydroxide, 1 prospective study found lower bone mineral content in aluminum hydroxide-treated patients. Minor, and inconsistent, differences were found. Because of the potential for neurotoxicity and osteomalacia that are associated with aluminum-containing phosphate binders,146,147,171 the use of these compounds should be reserved for patients with serum phosphorus greater than 7.0 mg/dL (2.26 mmol/L) and only for short-term therapy. However, the Work Group acknowledges that while there is morbidity associated with long-term aluminum intake, there is also increased mortality with phosphorus levels greater than 6.5 to 7.0 mg/dL (2.10 to 2.26 mmol/L). Thus, the 2 issues must be balanced. At the present time, there is no evidence that short-term use of aluminum-containing phosphate binders is associated with the development of aluminum bone disease or neurotoxicity. Therefore, the short-term (4 weeks) use of these compounds is not contraindicated. However, calcium citrate should be avoided while the patients receive aluminum-based compounds, since citrate increases the absorption of aluminum from the intestine171 and may precipitate acute aluminum toxicity.
In summary, the available evidence supports the hypothesis that all of the current phosphate binders are efficacious in controlling serum phosphorus levels. The majority of studies evaluated calcium-containing phosphate binders. However, recent studies on the use of the new nonaluminum-, nonmagnesium-, noncalcium-containing phosphate binder sevelamer was effective,83,152,157,172,173 and the Work Group felt that this agent has an important emerging role in the control of serum phosphorus in dialysis patients.
In CKD Stages 3 and 4 calcium levels are often low, contributing to secondary hyperparathyroidism. Furthermore, because these patients have some residual kidney function, phosphate and/or PTH control is usually achievable with lower doses of calcium-based phosphate binders. In CKD Stage 5, the current evidence and the opinion of the Work Group support the recommendation that the choice of phosphate binder should be determined by patient preference (number and size of binder, tablets or capsules), compliance, comorbid illnesses, side effects, cost, and the ability to control serum phosphorus levels while maintaining the desired calcium-phosphorus product (<55), and limiting the total calcium intake. However, the Work Group also recommends a noncalcium, nonmagnesium, nonaluminum phosphate binder as the therapy of choice in dialysis patients with low parathyroid hormone. The rationale for this recommendation is that these patients will usually have low-turnover bone disease, and the bone will be unable to incorporate a calcium load,174 predisposing to extraskeletal calcification. Also, calcium-based phosphate binders should not be used in patients with hypercalcemia or with severe vascular calcification (see below). In these situations, a noncalcium, nonmagnesium, nonaluminum phosphate binder should be used to control serum levels of phosphorus while avoiding excessive calcium intake.
The available data do not quantify an exact amount of calcium that could be given safely as a calcium-based phosphate binder. This is an important issue as recent studies suggest excessive calcium intake may worsen vascular and other extraskeletal calcification.87, 91,175 Additional data, that either did not fully meet the inclusion criteria or became available after the evidence report was completed, support this consensus of the Work Group. These data were reviewed by the Work Group and are summarized as follows.
In a cross-sectional study evaluating the presence of vascular calcification as assessed by electron-beam computed tomography scan in children and adolescents, the calcium-phosphorus product and prescribed calcium intake from phosphate binders were much higher in the patient group with calcification. In the group with calcification, the mean dose of prescribed binder was 6.456 g/day (elemental calcium/day), compared to 3.325 g/day in the no calcification group.87 Another cross-sectional study evaluating risks for significant vascular calcification assessed by ultrasound, found by multivariate analysis that the calcium load from phosphate binders was greater in those with calcification compared to those without calcification.91 There was a progressive increase from 1.35 ± 1.10 g/day of elemental calcium in patients with no calcification by ultrasound, to 1.50 ± 0.81 g/day in those with a calcification score of 2, and 2.18 ± 0.93 in those patients with a calcification score of 4 (P = 0.001 by ANOVA).91 Lastly, a prospective, randomized, controlled trial compared sevelamer HCl to calcium-based phosphate binders in 202 dialysis patients. The study compared the effect on serum phosphorus, calcium, calcium-phosphorus product, cholesterol and LDL levels, and aortic and coronary artery calcification evaluated by electron-beam tomography. Sevelamer and calcium-based phosphate binders achieved control of serum phosphorus levels similar to the recommended KDOQI levels; calcium-phosphorus product was slightly higher in the calcium-treated group. There were more hypercalcemic episodes and more suppression of PTH in the calcium-treated group. Blood levels of cholesterol and LDL were significantly lower in the sevelamer-treated group. In the 80% of patients with calcification at baseline, there was significant progression in aortic and coronary artery calcification in the calcium-treated group, but no progression in the sevelamer-treated group. In the calcium arm, the average dose of calcium acetate was 4.6 g/day (1,183 mg elemental calcium per day). The average dose of calcium carbonate was 3.9 g (1,560 mg elemental calcium). It should be cautioned that the observed results could be due to calcium load or lowering LDL cholesterol. However, taken together, these studies support the conclusion that calcium intake from phosphate binders should be limited in CKD patients on dialysis (Stage 5) to under 1,500 mg/day, and possibly lower.
The total calcium intake from diet, calcium-containing phosphate binders, and dialysate ideally should be equal to the recommended daily adequate intake (AI) for adults (1,000 to 1,500 mg/day). Given that the daily dietary intake of calcium for most dialysis patients is only 500 mg due to the restricted phosphorus diet, this leaves only 500 to 1,000 mg elemental calcium from calcium-containing phosphate binders. However, the Work Group recognizes the overwhelming importance of controlling serum phosphorus levels, which can rarely be done with calcium-containing phosphate binders while adhering to this limited daily calcium intake. Based on this and the above data, the Work Group recommends that the amount of calcium provided by calcium-based phosphate binders and diet should not exceed 2 g/day. This recommendation is not evidence-based and thus the clinician must individualize therapy taking into account cost, other vascular risk factors, and the patient’s tolerance of calcium-containing binders. For further discussion of the issue of daily calcium intake in CKD patients, see the discussion in the section, "Strength of Evidence" in Guideline 6.
For those patients who are on calcium-containing phosphate binders in amounts exceeding 2,000 mg total elemental calcium content, the Work Group strongly recommends adding a noncalcium, nonmagnesium, nonaluminum phosphate binder to decrease the total calcium intake. Sevelamer is such an agent currently available. It has the additional advantage of decreasing the serum levels of LDL cholesterol.
Obviously, the best phosphate binders are those that the patient will take consistently and as prescribed while limiting total calcium intake. As stated earlier in this document, the ability to adequately control serum phosphorus rests on appropriate education, patient compliance, and the use of tolerable phosphate binders. The latter needs to be individualized for patients and thus will require continuous monitoring with renal dietitians.
Longitudinal studies evaluating phosphate binders and their efficacy, side effects, and impact on morbidity and mortality are needed. Although the recently completed study demonstrated an advantage of sevelamer HCl compared to calcium-based phosphate binders in preventing progression of aortic and coronary arteries calcification, studies evaluating this positive effect on cardiovascular morbidity and mortality in dialysis patients are needed.