In CKD Patients (Stages 3 and 4):
6.1 The serum levels of corrected total calcium should be maintained within the "normal" range for the laboratory used. (EVIDENCE)
In CKD Patients With Kidney Failure (Stage 5):
6.2 Serum levels of corrected total calcium should be maintained within the normal range for the laboratory used, preferably toward the lower end (8.4 to 9.5 mg/dL [2.10 to 2.37 mmol/L]). (OPINION)
6.3 In the event corrected total serum calcium level exceeds 10.2 mg/dL (2.54 mmol/L), therapies that cause serum calcium to rise should be adjusted as follows:
6.3a In patients taking calcium-based phosphate binders, the dose should be reduced or therapy switched to a noncalcium-, nonaluminum-, nonmagnesium-containing phosphate binder. (OPINION) See Guideline 5.
6.3b In patients taking active vitamin D sterols, the dose should be reduced or therapy discontinued until the serum levels of corrected total calcium return to the target range (8.4 to 9.5 mg/dL [2.10 to 2.37 mmol/L]). (OPINION) See Guideline 8B.
6.3c If hypercalcemia (serum levels of corrected total calcium >10.2 mg/dL [2.54 mmol/L]) persists despite modification of therapy with vitamin D and/or discontinuation of calcium-based phosphate binders, dialysis using low dialysate calcium (1.5 to 2.0 mEq/L) may be used for 3 to 4 weeks (OPINION) See Guideline 9.
In CKD Patients (Stages 3 to 5):
6.4 Total elemental calcium intake (including both dietary calcium intake and calcium-based phosphate binders) should not exceed 2,000 mg/day. (OPINION) See Guideline 5.
6.5 The serum calcium-phosphorus product should be maintained at <55 mg2/dL2. (EVIDENCE) This is best achieved by controlling serum levels of phosphorus within the target range. (OPINION) See Guidelines 3, 4, and 5.
6.6 Patients whose serum levels of corrected total calcium are below the lower limit for the laboratory used (<8.4 mg/dL [2.10 mmol/L]) should receive therapy to increase serum calcium levels if:
6.6a There are clinical symptoms of hypocalcemia such as paresthesia, Chvostek’s and Trousseau’s signs, bronchospasm, laryngospasm, tetany, and/or seizures (OPINION); or
6.6b The plasma intact PTH level is above the target range for the CKD Stage (See Table 15 in Guideline 1). (OPINION)
6.7 Therapy for hypocalcemia should include calcium salts such as calcium carbonate (EVIDENCE) and/or oral vitamin D sterols. (EVIDENCE) See Guideline 8B.
Maintenance of normal calcium balance and serum calcium levels depend on integrated regulation of calcium absorption and secretion by the intestinal tract, the excretion of calcium by the kidney, and calcium release from and calcium deposition into bone. Parathyroid hormone, by stimulating bone resorption and kidney distal tubular calcium reabsorption in the kidney, and activating renal hydroxylation of 25(OH)D3 to 1,25(OH)2D3 increases serum calcium levels. Depression in serum levels of calcium by itself stimulates, through the calcium-sensing receptor (CaR) in the parathyroid gland, the secretion of preformed PTH from parathyroid gland within seconds. Subsequently, PTH biosynthesis by parathyroid gland increases over 24 to 48 hours and, if persistent, is followed by parathyroid gland hypertrophy and hyperplasia. Vitamin D metabolites and serum phosphorus levels also regulate PTH levels in blood. These homeostatic mechanisms are distorted in early stages of CKD and continue to deteriorate as loss of kidney function progresses.
The adult human body contains approximately 1,300 g of calcium with 99% in skeleton, 0.6% in soft tissues, and 0.1% in extracellular fluid.176 Normal values for serum total calcium concentration vary among clinical laboratories, depending on the methods of measurement, with a normal range being 8.6 to 10.3 mg/dL (2.15 to 2.57 mmol/L) for adults.177,178 Variations in serum levels of calcium depending on age and gender have been observed.179 Calcium in blood exists in three distinct fractions: protein-bound calcium (40%), free (formerly called ionized) calcium (48%), and calcium complexed with various anions such as phosphate, lactate, citrate, and bicarbonate (12%). Free calcium can be measured using ion-selective electrodes in most hospitals and values in adults range between 4.65 and 5.28 mg/dL (1.16 and 1.32 mmol/L).177,178 Free calcium should be assessed if subtle changes are expected or total calcium measurements are not adequate. Generally, reproducibility of free calcium measurement is worse than those of total calcium; the technique is time-consuming and more expensive than total calcium measurements. For these reasons, and because free calcium is not routinely measured, this Guideline will be based on the levels of total calcium in the blood. The latter does reflect the measured levels of free calcium if plasma levels of protein are normal. If plasma levels of albumin are low, a correction of the measured serum levels of calcium should be made. Several formulas have been developed to correct total calcium for abnormal albumin or to calculate free calcium both in healthy subjects and patients with CKD, but all of them are encumbered with limitations (see Guideline 6, Rationale). Also, a fall in pH of 0.1 unit will cause approximately a 0.1 mEq/L rise in the concentration of ionized calcium since hydrogen ion displaces calcium from albumin, whereas alkalosis decreases free calcium by enhancing binding of calcium to albumin.179
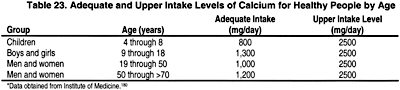
There are no biochemical measurements that reflects calcium nutritional status in normal subjects and in patients with kidney disease. The major indirect measures of calcium adequacy are skeletal health assessed by risk of fractures, bone mass measurements, and desirable rates of calcium retention in bone. Based on these surrogate markers, the Dietary Reference Intake (DRI) Committee180 recommended the term "adequate intakes" (AIs) of calcium. This represents an approximation of the calcium intake that, in the judgment of the DRI Committee, is sufficient to maintain calcium nutriture based on observed or experimentally determined estimates of average calcium intake by groups of healthy people. The recommended dietary allowance (RDA), the term used for average daily dietary intake level that is sufficient to meet the nutritional requirements of 97% to 98% of all healthy individuals in a life stage and gender group, could not be established. At the same time, the tolerable upper level for calcium intake was established; this represents the maximal intake of calcium that is likely to pose no risks of adverse effects in healthy individuals. The examples of adequate intake and upper intake levels of calcium in various age groups of healthy subjects are presented in Table 23.
The total daily intake of elemental calcium in CKD patients should not exceed 2,000 mg per day. Table 24 provides the calcium content of various commercially available calcium-based binders.
Adequate dietary intake of calcium in patients with different stages of CKD is more difficult to estimate than in healthy subjects when one takes into consideration the changes in calcium, phosphorus, vitamin D, PTH, and bone metabolism that occur in CKD. Ideal dietary calcium intake should provide enough calcium to maintain calcium balance as close as possible to that of the age- and gender-matched healthy population. Calcium balance (intake minus the sum of all losses) in the healthy population is generally positive (+200 mg to +300 mg/day) during adolescence, slightly positive (10 to 50 mg/day) at age 19 through 30, neutral in mature adults, and becomes negative at advanced age.180 Whether negative calcium balance in the healthy aged population is the optimal status is a question for debate.
Additionally, in CKD patients, the fraction of intestinal calcium absorption in the duodenum and jejunum is reduced103 because this process is vitamin D-dependent,181 and CKD patients have reduced blood levels of 1,25(OH)2D.182 However, passive intestinal calcium absorption which is gradient-dependent can be augmented by increasing calcium intake.181
Patients with CKD who are treated with metabolites of vitamin D or calcium supplementation are particularly prone to develop hypercalcemia. This complication occurs especially in those with low-turnover bone disease. The clinical presentation of hypercalcemia varies from a mild, asymptomatic, biochemical abnormality detected during routine screening to a life-threatening emergency.183,184
Hypercalcemia, together with hyperphosphatemia, or each individually can be responsible for increased blood Ca-P product. Since serum phosphorus levels in patients with CKD are usually increased by a higher factor (from 3.5 mg/dL [1.13 mmol/L] to 7 mg/dL [2.26 mmol/L], giving a factor of 2), compared to calcium (from 9.5 mg/dL [2.37 mmol/L] to 11 mg/dL [2.74 mmol/L], giving a factor of 1.2), the relative importance of serum phosphorus levels in generating higher Ca-P product, expressed as mg2/dL2, is greater than the serum calcium levels. Still, the serum calcium levels could be critical185 if the serum phosphorus levels are very high, which is indeed the case in patients with Stage 5 CKD.
In the presence of high Ca-P product in blood, soft-tissue calcification is likely but not always associated with high Ca-P product, since many factors are involved in the genesis of soft-tissue calcification (Table 6).
It is important that patients with CKD have normal serum levels of corrected total calcium, since chronic lower levels of calcium cause secondary hyperparathyroidism, have adverse effects on bone mineralization, and may be associated with increased mortality. Therefore, hypocalcemia should be treated. Also, adequate calcium intake in CKD patients is needed to prevent negative calcium balance and since dietary intake of calcium in CKD patients is restricted, calcium supplementation may be required. At the same time, high calcium intake should be avoided since patients with CKD may encounter difficulties in buffering increased calcium loads, and such difficulty may result in hypercalcemia and/or soft-tissue calcification. Indeed, hypercalcemia is a frequent occurrence during therapy with calcium-based phosphate binders and/or active vitamin D sterols. Spontaneous hypercalcemia also occurs in CKD patients.
It is accepted that total calcium levels need to be adjusted for the level of albumin to better reflect the free calcium.179 The Evidence Report of these Guidelines cites 2 major studies that evaluated various formulas for correction of total calcium for albumin in 82 hemodialysis and 34 continuous ambulatory peritoneal dialysis (CAPD) patients.186,187 One of these studies186 used preferable statistical methods and also employed strict control of blood drawing and handling. Albumin was assayed by an automated bromocresol green method (BCG), total calcium by arenazo III binding, and ionized calcium by ion-selective electrode. Therefore, the equation derived from this study most closely approximates corrected total calcium in patients with CKD with an interclass correlation value of 0.84:
Corrected calcium (mg/dL) = Total calcium (mg/dL) + 0.0704 × [34 - Serum albumin (g/L)]
The use of different methods for measuring either albumin or calcium may yield different correlations from the one derived from this study. For the routine clinical interpretation of serum calcium needed for appropriate care of patients with kidney diseases, a simple formula, which yields similar results, for adjusting total serum calcium concentration for changes in plasma albumin concentration, can be used by clinicians179:
Corrected total calcium (mg/dL) = Total calcium (mg/dL) + 0.8 × [4 - Serum albumin (g/dL)]
Patients with GFR below 60 mL/min/1.73 m2 (Stage 3 CKD) usually, but not invariably, show a detectable decrease in the blood levels of total and free calcium.31,188 The serum calcium levels decrease further as kidney function deteriorates. In advanced stages of CKD, the fraction of total calcium bound to complexes is increased189; thus, free (ionized) calcium levels are decreased despite normal total serum calcium levels. Acidosis, on the other hand, may increase the serum levels of free calcium. With initiation of regular hemodialysis, the levels of serum total calcium usually normalize.
Hypocalcemia as a risk factor for negative outcomes such as increased mortality, incidence of fractures and bone disease, and quality of life was not adequately addressed in reported clinical studies. A few studies published in the early 1970s suggest that hypocalcemia may have detrimental consequences for patients with CKD.104,105,108,190-192 In 1 cohort study, 433 patients beginning dialysis therapy were followed prospectively for an average of 41 months.193 In 281 of the patients, the level of total calcium was <8.8 mg/dL. After adjusting for comorbid conditions, plasma albumin and blood hemoglobin, chronic hypocalcemia was associated with increased mortality (P < 0.006). This association was similar among patients treated with hemodialysis or peritoneal dialysis. Covariant analysis showed that hypocalcemia in these patients was associated with de novo and recurrent cardiac ischemic heart disease and congestive heart failure.
A positive relationship has been found between serum calcium level and mineralization surface and osteoid surface,105 and a statistically significant relationship between the serum calcium level and the percentage of metacarpal cortical/total bone area assessed by X-ray.108 However, this was not the case when the cortical area of bone in the patients was calculated as a percentage of cortical area of bone in normal subjects.104 Serum levels of total alkaline phosphatase activity, used as a marker of the severity of secondary hyperparathyroidism in patients with CKD, did not correlate with the serum levels of calcium.104,105,108,190-192 Despite moderate significant inverse correlation between serum calcium levels and serum PTH levels,190,191 it was not possible to calculate the relative risk for development of secondary hyperparathyroidism for particular levels of serum calcium. Some of the newer studies45 did not find a relationship between elevated serum levels of PTH observed in CKD patients with different levels of GFR and the levels of serum calcium, which were within the normal range independent of the stage of kidney disease.
Taken together, the results of the Evidence Report with regard to this Guideline indicate that hypocalcemia is a risk for bone disease and for development of secondary hyperparathyroidism and/or increased risk of mortality. Thus, the detection of true hypocalcemia and its appropriate treatment is important for management of patients with CKD.
There are no data suggesting that transient mild hypercalcemia has detrimental effects on morbidity in patients with CKD. In 1 study, there was no evidence that isolated hypercalcemia is associated with increased morbidity in the hemodialysis population.92 Hypercalcemia poses a risk for CKD patients as it would increase the Ca-P product index in blood. Severe hypercalcemia with clinical symptoms must be treated appropriately.
Net calcium absorption is reduced in chronic renal failure as a consequence of both decreased calcium intake and decreased fraction of calcium absorbed by the intestine. The fraction of intestinal absorption of calcium is decreased early in the course of kidney disease. This is observed in Stage 3 CKD and worsens as CKD progresses.103,194-197 Initiation of dialysis does not improve calcium absorption.195-197 It is common to observe significant variability in intestinal calcium absorption within a group of patients with the same degree of kidney dysfunction,103,194-197 and, therefore, population studies may not be adequate to address the status of intestinal calcium absorption in individual patients.
Dietary calcium intake is low in patients with CKD. Intake of calcium in adults with advanced CKD ranged between 300 and 700 mg/day195,198; in those treated with hemodialysis, calcium intake averaged 549 mg/day199; and it was 80% of the recommended daily allowance in children with GFR between 20 and 75 mL/min/1.73 m2.200 When dietary calcium intake was less than 20 mg/kg /day, patients with CKD had negative net intestinal calcium balance, but neutral calcium balance was achievable with calcium intake around 30 mg/kg/day.201
There are no data on calcium retention as a function of increased long-term calcium intake in patients with CKD that are similar to data calculated for healthy adolescents, young adults, and adult men, which shows that calcium retention reaches a plateau despite an increase in calcium intake from 1,000 to 2,500 mg/day.181 Thus, we are poorly equipped to establish values for adequate intake of calcium in patients with kidney disease. The opinion of the Work Group is that an intake of 2.0 g/day of calcium (dietary and supplements) is appropriate for CKD patients.
While this recommendation of the Work Group is not based on evidence provided in the Evidence Report, there are data from different studies identifying the requirement of calcium for various components of calcium balance (intestinal calcium absorption and calcium secretion) and calcium losses (urinary, fecal, and sweat) in CKD patients (Table 25). These data show that the requirement of daily calcium intake in Stage 3 CKD is 1.5 to 2.0 g/day and in Stages 4 and 5 CKD (patients not on dialysis), it is 1.5 to 1.8 g/day. The Work Group’s recommendation of total daily calcium intake of 2.0 g/day is in agreement with these data.
Furthermore, in dialysis patients, calcium supplementation of 3.0 g/day in addition to the 400 to 500 mg in dietary calcium resulted in hypercalcemia in up to 36% of patients.203 Other studies show lower, but still significant, incidences of hypercalcemia during high calcium intake.141,204 This clearly suggests that there is a tolerable upper intake level for patients with CKD and, therefore, higher daily calcium intake (>2.0 g/day) should be avoided.
The effectiveness of different calcium salts used for calcium supplementation was partially addressed by 4 studies.41,173,205,206 Only 1 of these studies206 directly compared the efficacy of 2 different calcium salts (calcium carbonate versus calcium citrate). However, this study followed the patients for only 3 hours after administration of the calcium supplements, and therefore the results represent only short-term effects. The other 3 studies compared the use of calcium carbonate to placebo or no calcium supplement. Because of the different study conditions and patient populations, and because these studies did not directly address the question being asked, it was not useful to conduct a meta-analysis. Therefore, the recommendation for the use of calcium carbonate for calcium supplementation in this Guideline is opinion-based and endorsed by the Work Group.
Similarly, the 4 studies cited above did not provide information that could be utilized to ascertain whether giving the calcium salts before, during, or after meals is more effective. Further, the data are not helpful in deciding whether it is better to give the calcium salts in 1 dose per day or divided into multiple doses.
The question as to when to initiate calcium supplementation during the course of CKD is not answered by the available data in the literature. Certainly, in the presence of overt hypocalcemia, calcium supplementation is indicated. However, determining when to initiate calcium therapy in patients with CKD involves a consideration of multi-dimensional biological parameters on the part of the clinician. It seems, however, that calcium supplementation should be considered in CKD patients when serum levels of PTH begin to rise, ie, GFR <60 mL/min/1.73 m2 (Stage 3 CKD).
An association was observed between Ca-P product and the risk of death in a random sample of the US population of 2,669 patients treated for at least 1 year with hemodialysis between 1990 and 1993.92 Patients with Ca-P product above 72 (20% of all patients) had a 34% higher relative risk of death compared to patients with Ca-P product in the range of 42 to 52.92 The increased risk was observed in proportion to the elevation of Ca-P product; indeed, for every increase of 10 in Ca-P product, there was an 11% increase in relative risk of death.
The Evidence Report cites 4 studies that address the issue of Ca-P product as a risk for soft-tissue calcification. One prospective, uncontrolled study of 137 patients showed that 35 patients ages 55 to 64, with poorly controlled Ca-P product levels (above 60) had increased aortic calcification index (ACI = 26.1) as compared to 20 patients of the same age with well-controlled Ca-P product <60 (ACI = 17.7).86 Another prospective, controlled study using stepwise discrimination analysis showed significantly higher risk for mitral annular calcification in hemodialysis patients with Ca-P product of 63 ± 13 compared to those with Ca-P product of 56 ± 13.207 An additional study showed that, in young adults on hemodialysis who had Ca-P product of 65 ± 10.6, coronary artery calcification was significantly higher than in those with Ca-P product of 56 ± 12.7.87 One retrospective, controlled study in CAPD patients showed no significant differences in Ca-P product levels in 17 patients with mitral annular calcification as compared to 118 patients without this abnormality.208 Despite the fact that these studies were not controlled for potential confounding variables and are encumbered with selection bias, it seems reasonable to conclude that high levels of Ca-P product can pose a risk of vascular calcification.
The level of Ca-P product in CKD patients at which risk for calcification is very low or unlikely to occur, has been debated over the last 40 years, but no strong evidence is available to answer this question. As discussed above, Ca-P products are most likely a risk for calcification, but assessing calcification risk does not involve arriving at "yes" or "no" answers. The theory is that calcification risk increases as Ca-P product increases; however, evidence on this relationship is scant and will be presented below.
Five studies207-211 examined some measure that evaluate Ca-P product as a risk for extraskeletal calcification. None examined risk for future calcification. All were cross-sectional studies. Three were retrospective208,210,211 and 2 prospective.207,209 They used different methods (radiography, scintigraphy, CT, echocardiography) for detection of calcification and examined different organs for calcification (eg, soft tissue, mitral and aortic valve, aorta, lung). Only 2 studies208,211 provided enough information to calculate risk ratios for Ca-P product for inducing soft-tissue calcification. One study208 included 135 Stage 5 CKD predialysis patients and 76 patients on CAPD, and the other211 reported on 47 patients for more than 2 years on CAPD. This limited information suggests that Ca-P product may not be a useful indicator of calcification in patients with Stage 5 CKD, as no trend for risk was seen.208 Data on patients treated with CAPD for 2 years showed that the risk for mitral calcification increased as Ca-P increased.211 In contrast, in patients treated with CAPD for 1 year, there was no relationship between the risk for calcification and the levels of Ca-P product.208 The confidence intervals in this small study are very wide, and thus firm conclusions cannot be reached. Neither of these studies examined whether Ca-P product can be used as a predictor of future calcification.
Two case-controlled studies indicated that there were significant differences in Ca-P product between patients with and without aortic valve calcification and mitral annular calcification207,210 and normal and abnormal visceral uptake of 99Tc-PP or 99Tc-MDP.209
The incidence of visceral calcification in a selected dialysis population was high when mean Ca-P product exceeded 68 and low when mean Ca-P product was 51.209 Frequent incidence of visceral calcification207 and mitral valve calcification210 was reported when Ca-P product exceeded 60 and calcification was unlikely when mean Ca-P product was around 50.209,210 It must be noted that a significant number of patients did not develop extraskeletal calcification despite a high Ca-P product.87,209,210
Thus, the available evidence is limited but convincing that primary outcome (increased death rate) and secondary outcome (extraskeletal calcification) are related to Ca-P product. If this product exceeds 55, there is increased risk for development of calcification and possibly increased risk for lower patient survival. Thus, the goal level of Ca-P product should be below 55.
There are no long-term epidemiological studies in patients with CKD to support the current recommendation on the "normal" blood calcium range for the CKD population, the amount of calcium supplementation, the time at which calcium supplementation should be initiated, or the type of calcium salts to be used.
The evidence that hypocalcemia is a risk factor for negative outcomes such as increased mortality, incidence of fracture and bone disease, and quality of life was not addressed in long-term studies of CKD patients, except for 1 study that followed mortality associated with chronic hypocalcemia (serum total calcium of 8.8 mg/dL [2.20 mmol/L]) for 4 years. There is no randomized, controlled trial suggesting that adequate calcium intake or calcium supplementation will improve bone mineralization, quality of life, and mortality.
Although an increased risk of mortality is associated with elevated Ca-P product, there are no data on the cause of death associated with the high Ca-P product.
This Guideline supports the use of corrected total serum calcium in the evaluation of the derangements of calcium, phosphorus, PTH, and bone in CKD patients. It must be recognized that limited data are available with regard to adequate calcium intake in Stages 3 and 4 CKD patients, and particularly in Stage 5 CKD patients. The avoidance of excessive exposure of CKD patients to calcium while maintaining adequate intake of calcium has significant clinical implications, and effort is needed to achieve this goal. Successful implementation will require increased time of the dietitian for the guidance and support of patients in this regard.
Further studies are required to find the appropriate reference range of serum calcium for different age, gender, and race in CKD patients and those treated with dialysis. Studies are needed to determine the appropriate calcium intake, the time in the course of CKD when calcium supplementation should be initiated, and the type of calcium salts to be used. Also, studies are needed to further understand the relationship between Ca-P product and morbidity and mortality.