This Guideline encompasses 2 parts: Guideline 8A, which deals with active vitamin D sterol therapy in CKD Stages 3 and 4, and Guideline 8B, which deals with CKD Stage 5.
GUIDELINE 8B. VITAMIN D THERAPY IN PATIENTS ON DIALYSIS (CKD STAGE 5)
8B.1 Patients treated with hemodialysis or peritoneal dialysis with serum levels of intact PTH levels >300 pg/mL (33.0 pmol/L) should receive an active vitamin D sterol (such as calcitriol, alfacalcidol, paricalcitol, or doxercalciferol; see Table 28) to reduce the serum levels of PTH to a target range of 150 to 300 pg/mL (16.5 to 33.0 pmol/L). (EVIDENCE)
8B.1a The intermittent, intravenous administration of calcitriol is more effective than daily oral calcitriol in lowering serum PTH levels. (EVIDENCE)
8B.1b In patients with corrected serum calcium and/or phosphorus levels above the target range (see Guidelines 3 and 6, respectively), a trial of alternative vitamin D analogs, such as paricalcitol or doxercalciferol may be warranted. (OPINION)
8B.2 When therapy with vitamin D sterols is initiated or the dose is increased, serum levels of calcium and phosphorus should be monitored at least every 2 weeks for 1 month and then monthly thereafter. The plasma PTH should be measured monthly for at least 3 months and then every 3 months once target levels of PTH are achieved. (OPINION)
8B.3 For patients treated with peritoneal dialysis, oral doses of calcitriol (0.5 to 1.0 µg) or doxercalciferol (2.5 to 5.0 µg) can be given 2 or 3 times weekly. Alternatively, a lower dose of calcitriol (0.25 µg) can be administered daily. (OPINION)
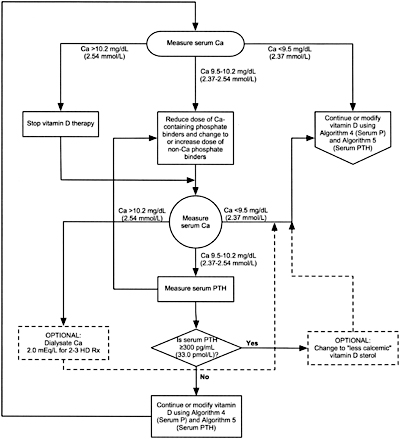
8B.4 When either hemodialysis or peritoneal dialysis patients are treated with active vitamin D sterols, management should integrate the changes in serum calcium, serum phosphorus, and plasma PTH. Each of these three variables is considered separately with suggested interventions based on the various values obtained in Algorithm 3, Algorithm 4, and Algorithm 5. (OPINION)
Algorithm 3. Managing Vitamin D sterols based on serum calcium levels.
Algorithm 4. Managing Vitamin D sterols based on serum phosphorus levels.
Algorithm 5. Managing Vitamin D sterols based on intact PTH levels.
Patients with CKD who undergo dialysis have reduced plasma levels of 1,25(OH)2D3. This leads to reduced intestinal absorption of calcium (thereby contributing to hypocalcemia) and impaired suppression of the parathyroid gene that initiates the synthesis of PTH. The result is secondary hyperparathyroidism that often progresses. Treatment with calcitriol or another active vitamin D sterol both reduces PTH secretion with resultant improvement of hyperparathyroid bone disease, and improves musculoskeletal symptoms, when these are present.
Fig 12. Meta-analysis of oral versus intravenous calcitriol on PTH suppression.
A major side-effect of vitamin D treatment is increased intestinal absorption of calcium and phosphorus; this can produce hypercalcemia and aggravate hyperphosphatemia. Treatment with active vitamin D sterols can also markedly lower serum levels of intact PTH and reduce bone formation strikingly; this can produce a condition with low bone turnover, termed adynamic bone disease. For these reasons, serum levels of calcium and phosphorus, and those of intact PTH, must be monitored during vitamin D therapy, and vitamin D therapy adjusted accordingly (Algorithm 3, Algorithm 4, and Algorithm 5).
Treatment of secondary hyperparathyroidism in end-stage kidney disease patients with oral or intravenous calcitriol, intravenous paricalcitol, oral or intravenous doxercalciferol, or oral or intravenous alfacalcidol can reduce the elevated levels of intact PTH,11,261-270 and may be useful to treat various clinical features of symptomatic secondary hyperparathyroidism.261,263,266 With such treatment, improved features of hyperparathyroid bone disease have been reported.262,263,271,272 Reductions of both plasma total alkaline phosphatase and/or bone-specific alkaline phosphatase, consistent with a reduction of the elevated bone turnover state, have been shown during treatment with several of these vitamin D preparations.11, 262-264,266,271,273
In dialysis patients who have not received vitamin D, or those who have received daily oral calcitriol in doses lower than 0.5 µg/day, serum levels of intact PTH correlate with the degree of secondary hyperparathyroidism33,34,274; moreover, patients with intact PTH levels <400 pg/mL (44.0 pmol/L) and normal (or low) serum levels of calcium, usually have only mild hyperparathyroidism.33,274 In these patients, the optimal control of serum phosphorus levels, combined with the use of calcium-based phosphate binders, may result in no further rise of serum PTH levels. When serum levels of intact PTH exceed 500 to 600 pg/mL (55.0 to 66.0 pmol/L), moderate or even severe hyperparathyroid bone disease is usual. When intact PTH levels exceed 1,000 pg/mL (110.0 pmol/L), larger doses of the vitamin D sterols are generally required.269, 275-278 During treatment with intravenous calcitriol275 or oral doxercalciferol269 in prospective trials, there is evidence that larger doses are required for the treatment of patients with severe secondary hyperparathyroidism compared to patients with less severe hyperparathyroidism. Moreover, the suppression of serum levels of intact PTH in patients with severe hyperparathyroidism may require treatment for longer periods of time, eg, more than 12 to 24 weeks.269,275-277 The reason for the delayed response of some patients is unclear; it might be related to upregulation of vitamin D receptors that are often reduced in the large nodular parathyroid glands in end-stage kidney disease patients with more severe secondary hyperparathyroidism.279
It is recommended that the dosage of a vitamin D sterol be adjusted in accordance with the severity of secondary hyperparathyroidism. The evidence that intact PTH levels correlate with the severity of bone disease in patients who have not received pulse-dose intravenous or oral calcitriol is quite good.33,34 However, the optimal doses of vitamin D sterols and the optimal serum levels of intact PTH that should be the target in patients who have received such therapy for longer than 6 to 12 months is less certain.
Several trials that were not placebo-controlled have shown the effectiveness of intermittent intravenous and intermittent oral calcitriol to suppress serum levels of intact PTH in patients undergoing hemodialysis,15,280 including some patients with severe hyperparathyroidism280-283; moreover, these results appeared more favorable than earlier experiences with daily oral dosing when reductions of dosage were commonly needed.284,285 However, the meta-analysis of four trials that compared intermittent intravenous calcitriol with oral calcitriol in randomized, controlled studies286,287 or cross-over trials288,289 indicated that intravenous therapy was more effective than oral treatment (either daily or "pulse" treatment) for the suppression of intact PTH levels (Fig 12). However, there are certain qualifications about the trials combined for this meta-analysis: Two trials compared daily oral treatment with thrice weekly intravenous treatment287,289; in the trial that studied patients with the highest pretreatment intact PTH levels, the oral "group" was a combination of one group randomly assigned to intermittent treatment and a second group assigned to daily therapy.290 The degree of hyperparathyroidism was very mild in 2 trials, as the entry intact PTH levels averaged less than 400 pg/mL (44.0 pmol/L).287,288 In 2 trials that prospectively compared intermittent oral and intravenous calcitriol in patients with more severe hyperparathyroidism,291,292 the numbers of patients completing the study was too small (n < 10) to meet the criteria for the meta-analysis. In patients with more severe hyperparathyroidism (trials with intravenous calcitriol that adjusted the dosage upward if PTH levels were not suppressed), the use of calcitriol doses below 0.75 to 1.0 µg per treatment were often less effective in lowering intact PTH levels.275,293 Moreover, the earlier placebo-controlled trials with daily oral calcitriol found that patients could rarely tolerate daily doses of 0.5 µg per day without developing hypercalcemia. 284,285
The results of oral trials with calcitriol that were not placebo-controlled lead to the conclusion that pulse or intermittent therapy yielded better results than were reported with daily therapy; meta-analysis of the results of 3 randomized, controlled trials that compared daily oral with intermittent oral calcitriol failed to show any superiority of intermittent therapy over daily therapy.265, 290,294 Two of these studies265,294 had patients with only mild hyperparathyroidism, and few patients entered into treatment with intact PTH levels above 600 pg/mL (66.0 pmol/L). Despite randomization of treatment in one study,294 each of the 5 patients having pretreatment intact PTH levels above 600 pg/mL (66.0 pmol/L) were assigned to intermittent therapy. In another study,290 the trial with the highest pretreatment intact PTH levels, the serum calcium levels were higher with daily than with intermittent therapy. Thus, conclusions about there being no difference depending on the frequency of dosing must be viewed with caution.
The major side effects of active vitamin D sterols, including calcitriol and alfacalcidol, are increases in the serum levels of calcium and phosphorus leading to hypercalcemia and worsening of hyperphosphatemia. These concerns have led to efforts to develop analogs of vitamin D which might have less calcemic and/or phosphatemic effects, while retaining efficacy for the suppression of high levels of PTH.295,296 Several such analogs are now in clinical use. Paricalcitol and doxercalciferol are available in the United States, and maxicalcitol and falecalcitol are available in Asia.11,270,297,298 Extensive data in normal animals and in experimental animals with uremia have demonstrated that maxicalcitol and paricalcitol are less calcemic and phosphatemic than calcitriol and yet retain effectiveness in suppressing PTH.299-301 Studies in vitamin D-deficient animals with doxercalciferol have demonstrated no difference in calcium or phosphorus absorption from the intestine and in changes in serum calcium compared to alfacalcidol, but doxercalciferol was associated with a decreased mortality in toxicology studies.302,303 Additional studies have shown that doxercalciferol is associated with less calciuria than alfacalcidol.304,305 Definitive quantitative data comparing these vitamin D sterols to calcitriol or to each other in controlled clinical trials are not available at the present time.
In placebo-controlled trials with calcitriol, alfacalcidol, paricalcitol, and doxercalciferol, there were increments of serum phosphorus during treatment,11, 269,306-309 and analysis indicated no difference between the sterols regarding their effects on raising serum levels of phosphorus. Treatment with vitamin D should not be undertaken or continued if serum phosphorus levels exceed 6.5 mg/dL, because of this risk of further elevating serum phosphorus levels.
Another side-effect of intermittent treatment with an active vitamin D sterol is the appearance of subnormal bone formation, with "adynamic" or "aplastic" bone.62,310 In end-stage kidney disease patients who had not received pulse doses of calcitriol and had intact PTH levels below 150 pg/mL (16.5 pmol/L), there was a high incidence of subnormal bone formation on bone biopsy, with "adynamic" or "aplastic" bone.33 When intact PTH levels are below 65 pg/mL (7.15 pmol/L), the occurrence of adynamic bone is nearly universal.26,33 Mild hyperparathyroid bone disease may be preferable to adynamic bone because of the loss of the capacity of bone buffering for the added extracellular calcium;174 this likely accounts for the increased risk of hypercalcemia in patients with adynamic bone.14,62 Also, there may be increased risk of vascular calcification in patients with biochemical features that are consistent with adynamic bone.91 In adolescents and young adults with end-stage kidney disease, adynamic bone310 and even reduced linear growth occurred in association with intermittent calcitriol therapy when the intact PTH levels were reduced below 400 to 450 pg/mL (44.0 to 49.5 pmol/L).311 Reported observations of the development of adynamic bone in adult end-stage kidney disease patients in association with pulse therapy with calcitriol are limited to a small number312; however, there is little reason to believe that the bone of adults would not show the effects observed in pediatric-age patients.
When one elects to observe dialysis patients with intact PTH levels <600 pg/mL (66.0 pmol/L) without initiating vitamin D therapy, serial intact PTH levels should be monitored. If the levels show a progressive rise, treatment should be initiated.
Many of the studies cited above with calcitriol and alfacalcidol that originated before 1980 lacked parallel control groups,261-264,266, 271,272,313-315 and the assays for PTH were variable and some involved PTH fragments261-264,266 that are cleared by the kidney; thus, comparison with the current trials that utilize so-called "intact PTH" is not possible. Also, many patients in the early trials had "severe" and symptomatic bone disease, findings that have become more rare with better control of secondary hyperparathyroidism. With studies of the "newer" vitamin D sterols, such as falecalcitriol, paricalcitol, and doxercalciferol, there were often parallel controls. 11,268,269,298 However, the severity of secondary hyperparathyroidism was mild to moderate, based on pretreatment serum levels of intact PTH, in most patients entered into trials with falecalcitriol268,298 or paricalcitol.11 For these reasons, comparison of data with the different vitamin D sterols must be regarded as tentative, particularly for patients with severe secondary hyperparathyroidism, defined as serum levels of intact PTH >1,200 pg/mL (132.0 pmol/L). Also, it is almost certain that such patients would be considered inappropriate for a long-term, placebo-controlled trial.
The conclusions that pulse intravenous therapy is better then pulse oral treatment must also be regarded as tentative; similarly, the conclusions that daily oral therapy is as effective as pulse oral therapy given 2 or 3 times a week may only apply to patients with mild secondary hyperparathyroidism for the reasons noted above.
Secondary hyperparathyroidism and hyperparathyroid high-turnover bone disease in CKD are treatable abnormalities with active vitamin D sterols. There are many of these sterols available and others are being developed. Since one of the side-effects of the therapy with these sterols is hypercalcemia, one would want to use a sterol effective in treatment of the bone disorder with less or no hypercalcemia.
Trials that compare different vitamin D sterols in patients with end-stage kidney disease are needed. Also, prospective trials are needed to evaluate the effect of pulse-dose calcitriol or other vitamin D sterol on bone, with study of the relationship between serum levels of intact PTH and bone turnover using double tetracycline, to assess a possibly important side-effect of vitamin D treatment. Moreover, little is known about the ideal target for serum levels of intact PTH during treatment with vitamin D. It is possible that the incidence of adynamic bone will increase substantially if vitamin D sterols are employed in patients who have only modest elevations of intact PTH levels. Studies are needed to examine the value of bone markers and to assess the relationship between the so-called "whole PTH molecule," "intact PTH," and bone histomorphometry during vitamin D treatment. Large studies that evaluate fracture rates should include data on previous vitamin D therapy in an effort to identify whether vitamin D treatment can modify the high incidence of fractures noted in end-stage kidney disease patients.