NKF KDOQI GUIDELINES
KDOQI Clinical Practice Guidelines for Bone Metabolism and Disease in Chronic Kidney Disease
GUIDELINE STATEMENTS
GUIDELINE 1. EVALUATOIN OF CALCIUM AND PHOSPHORUS METABOLISM
1.1 Serum levels of calcium, phosphorus, and intact plasma parathyroid hormone (PTH) should be measured in all patients with CKD and GFR <60 mL/min/1.73 m2. (EVIDENCE) The frequency of these measurements should be based on the stage of chronic kidney disease (Table 14). (OPINION)

1.2 These measurements should be made more frequently if the patient is receiving concomitant therapy for the abnormalities in the serum levels of calcium, phosphorus, or PTH, as detailed in Guidelines 4, 5, 7, and 8, and in transplant recipient, Guideline 16.
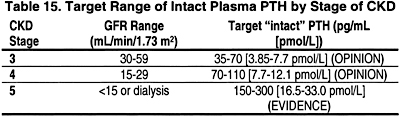
1.3 Measurement of plasma PTH levels may be done less frequently for those with levels within the low end of the target levels (Table 15). (OPINION)
1.4 The target range of plasma levels of intact PTH in the various stages of CKD are denoted in Table 15.
GUIDELINE 2. ASSESSMENT OF BONE DISEASE ASSOCIATED WITH CKD
2.1 The most accurate diagnostic test for determining the type of bone disease associated with CKD is iliac crest bone biopsy with double tetracycline labeling and bone histomorphometric analysis. (EVIDENCE)
2.2 It is not necessary to perform bone biopsy for most situations in clinical practice. However, a bone biopsy should be considered in patients with kidney failure (Stage 5) who have:
2.2a Fractures with minimal or no trauma (pathological fractures); (OPINION)
2.2b Intact plasma PTH levels between 100 and 500 pg/mL (11.0 to 55.0 pmol/L) (in CKD Stage 5) with coexisting conditions such as unexplained hypercalcemia, severe bone pain, or unexplained increases in bone alkaline phosphatase activity; (OPINION)
2.2c Suspected aluminum bone disease, based upon clinical symptoms or history of aluminum exposure. (OPINION) (See Guideline 11)
2.3 Bone radiographs are not indicated for the assessment of bone disease of CKD, (EVIDENCE) but they are useful in detecting severe peripheral vascular calcification (OPINION) and bone disease due to ß2 microglobulin amyloidosis. (See Guideline 10) (EVIDENCE)
2.4 Bone mineral density (BMD) should be measured by dual energy X-ray absorptiometry (DEXA) in patients with fractures and in those with known risk factors for osteoporosis. (OPINION)
GUIDELINE 3. EVALUATION OF SERUM PHOSPHORUS LEVELS
3.1 In CKD patients (Stages 3 and 4), the serum level of phosphorus should be maintained at or above 2.7 mg/dL (0.87 mmol/L) (EVIDENCE) and no higher than 4.6 mg/dL (1.49 mmol/L). (OPINION)
3.2 In CKD patients with kidney failure (Stage 5) and those treated with hemodialysis or peritoneal dialysis, the serum levels of phosphorus should be maintained between 3.5 to 5.5 mg/dL (1.13 to 1.78 mmol/L). (EVIDENCE)
GUIDELINE 4. RESTRICTION OF DIETARY PHOSPHORUS IN PATIENTS WITH CKD
4.1 Dietary phosphorus should be restricted to 800 to 1,000 mg/day (adjusted for dietary protein needs) when the serum phosphorus levels are elevated >4.6 mg/dL (1.49 mmol/L) at Stages 3 and 4 of CKD, (OPINION) and >5.5 mg/dL (1.78 mmol/L) in those with kidney failure (Stage 5). (EVIDENCE)
4.2 Dietary phosphorus should be restricted to 800 to 1,000 mg/day (adjusted to dietary protein needs) when the plasma levels of intact PTH are elevated above target range of the CKD Stage (see Table 15 in Guideline 1). (EVIDENCE)
4.3 The serum phosphorus levels should be monitored every month following the initiation of dietary phosphorus restriction. (OPINION)
GUIDELINE 5. USE OF PHOSPHATE BINDERS IN CKD
In CKD Patients (Stages 3 and 4):
5.1 If phosphorus or intact PTH levels cannot be controlled within the target range (see Guidelines 1, 3), despite dietary phosphorus restriction (see Guideline 4), phosphate binders should be prescribed. (OPINION)
5.2 Calcium-based phosphate binders are effective in lowering serum phosphorus levels (EVIDENCE) and may be used as the initial binder therapy. (OPINION)
In CKD Patients with Kidney Failure (Stage 5):
5.3 Both calcium-based phosphate binders and other noncalcium-, nonaluminum-, and nonmagnesium-containing phosphate-binding agents (such as sevelamer HCl) are effective in lowering serum phosphorus levels (EVIDENCE) and either may be used as the primary therapy. (OPINION)
5.4 In dialysis patients who remain hyperphosphatemic (serum phosphorus >5.5 mg/dL [1.78 mmol/L]) despite the use of either of calcium-based phosphate binders or other noncalcium-, nonaluminum-, nonmagnesium-containing phosphate-binding agents, a combination of both should be used. (OPINION)
5.5 The total dose of elemental calcium provided by the calcium-based phosphate binders should not exceed 1,500 mg/day (OPINION), and the total intake of elemental calcium (including dietary calcium) should not exceed 2,000 mg/day. (OPINION)
5.6 Calcium-based phosphate binders should not be used in dialysis patients who are hypercalcemic (corrected serum calcium of >10.2 mg/dL [2.54 mmol/L]), or whose plasma PTH levels are <150 pg/mL (16.5 pmol/L) on 2 consecutive measurements. (EVIDENCE)
5.7 Noncalcium-containing phosphate binders are preferred in dialysis patients with severe vascular and/or other soft-tissue calcifications. (OPINION)
5.8 In patients with serum phosphorus levels >7.0 mg/dL (2.26 mmol/L), aluminum-based phosphate binders may be used as a short-term therapy (4 weeks), and for one course only, to be replaced thereafter by other phosphate binders. (OPINION) In such patients, more frequent dialysis should also be considered. (EVIDENCE)
GUIDELINE 6. SERUM CALCIUM AND CALCIUM-PHOSPHORUS PRODUCT
In CKD Patients (Stages 3 and 4):
6.1 The serum levels of corrected total calcium should be maintained within the “normal” range for the laboratory used. (EVIDENCE)
In CKD Patients With Kidney Failure (Stage 5):
6.2 Serum levels of corrected total calcium should be maintained within the normal range for the laboratory used, preferably toward the lower end (8.4 to 9.5 mg/dL [2.10 to 2.37 mmol/L]). (OPINION)
6.3 In the event corrected total serum calcium level exceeds 10.2 mg/dL (2.54 mmol/L), therapies that cause serum calcium to rise should be adjusted as follows:
6.3a In patients taking calcium-based phosphate binders, the dose should be reduced or therapy switched to a noncalcium-, nonaluminum-, nonmagnesium-containing phosphate binder. (OPINION) See Guideline 5.
6.3b In patients taking active vitamin D sterols, the dose should be reduced or therapy discontinued until the serum levels of corrected total calcium return to the target range (8.4 to 9.5 mg/dL [2.10 to 2.37 mmol/L]). (OPINION) See Guideline 8B.
6.3c If hypercalcemia (serum levels of corrected total calcium >10.2 mg/dL [2.54 mmol/L]) persists despite modification of therapy with vitamin D and/or discontinuation of calcium-based phosphate binders, dialysis using low dialysate calcium (1.5 to 2.0 mEq/L) may be used for 3 to 4 weeks. (OPINION) See Guideline 9.
In CKD Patients (Stages 3 to 5):
6.4 Total elemental calcium intake (including both dietary calcium intake and calcium-based phosphate binders) should not exceed 2,000 mg/day. (OPINION) See Guideline 5.
6.5 The serum calcium-phosphorus product should be maintained at <55 mg2/dL2. (EVIDENCE) This is best achieved by controlling serum levels of phosphorus within the target range. (OPINION) See Guidelines 3,4, and5.
6.6 Patients whose serum levels of corrected total calcium are below the lower limit for the laboratory used (<8.4 mg/dL [2.10 mmol/L]) should receive therapy to increase serum calcium levels if:
6.6a There are clinical symptoms of hypocalcemia such as paresthesia, Chvostek’s and Trousseau’s signs, bronchospasm, laryngospasm, tetany, and/or seizures (OPINION); or
6.6b The plasma intact PTH level is above the target range for the CKD Stage. (See Table 15 in Guideline 1.) (OPINION)
6.7 Therapy for hypocalcemia should include calcium salts such as calcium carbonate (EVIDENCE) and/or oral vitamin D sterols. (EVIDENCE) See Guideline 8B.
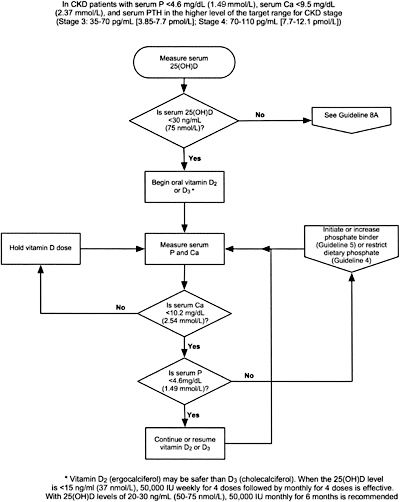
GUIDELINE 7. PREVENTION AND TREATMENT OF VITAMIN D INSUFFICIENCY AND VITAMIN D DEFICIENCY IN CKD PATIENTS (ALGORITHM 1)
In CKD Patients (Stages 3 and 4):
7.1 If plasma intact PTH is above the target range for the stage of CKD (Table 15, Guideline 1) serum 25-hydroxyvitamin D should be measured at first encounter. If it is normal, repeat annually. (EVIDENCE)
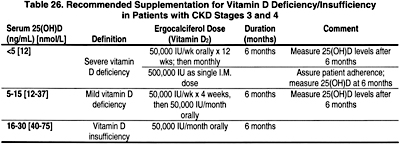
7.2 If the serum level of 25-hydroxyvitamin D is <30 ng/mL, supplementation with vitamin D2 (ergocalciferol) should be initiated (Table 26). (OPINION)
7.3 Following initiation of vitamin D therapy:
7.3a The use of ergocalciferol therapy should be integrated with the serum calcium and phosphorus (Algorithm 1).
7.3b The serum levels of corrected total calcium and phosphorus should be measured at least every 3 months. (OPINION)
7.3c If the serum levels of corrected total calcium exceeds 10.2 mg/dL (2.54 mmol/L), discontinue ergocalciferol therapy and all forms of vitamin D therapy. (OPINION)
Algorithm 1. Vitamin D supplementation in CKD (Stages 3 and 4)
7.3d If the serum phosphorus exceeds 4.6 mg/dL, add or increase the dose of phosphate binder. (See Guidelines 4 and 5.) If hyperphosphatemia persists, discontinue vitamin D therapy. (OPINION)
7.3e Once patients are replete with vitamin D, continued supplementation with a vitamin-D–containing multi-vitamin preparation should be used with annual reassessment of serum levels of 25-hydroxyvitamin D, and the continued assessment of corrected total calcium and phosphorus every 3 months. (OPINION)
In CKD Patients With Kidney Failure (Stage 5):
7.4 Therapy with an active vitamin D sterol (calcitriol, alfacalcidol, paricalcitol, or doxercalciferol) should be provided if the plasma levels of intact PTH are >300 pg/mL. (OPINION) See Guideline 8B.
GUIDELINE 8. VITAMIN D THERAPY IN CKD PATIENTS
This Guideline encompasses 2 parts: Guideline 8A, which deals with active vitamin D sterol therapy in CKD Stages 3 and 4, and Guideline 8B, which deals with CKD Stage 5.
GUIDELINE 8A. ACTIVE VITAMIN D THERAPY IN STAGES 3 AND 4 CKD (ALGORITHM 2)
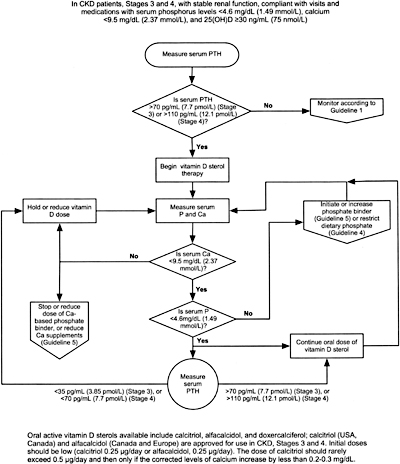
8A.1 In patients with CKD Stages 3 and 4, therapy with an active oral vitamin D sterol (calcitriol, alfacalcidol, or doxercalciferol) is indicated when serum levels of 25(OH)-vitamin D are >30 ng/mL (75 nmol/L) and plasma levels of intact PTH are above the target range for the CKD stage (see Table 15, Guideline 1). (EVIDENCE) The initial doses are provided in Table 27.

Algorithm 2. Management of CKD Patients (Stages 3 and 4) with Vitamin D sterols.
8A.1a Treatment with an active vitamin D sterol should be undertaken only in patients with serum levels of corrected total calcium <9.5 mg/dL (2.37 mmol/L) and serum phosphorus <4.6 mg/dL (1.49 mmol/L). (OPINION)
8A.1b Vitamin D sterols should not be prescribed for patients with rapidly worsening kidney function or those who are noncompliant with medications or follow-up. (OPINION)
8A.2 During therapy with vitamin D sterols, serum levels of calcium and phosphorus should be monitored at least every month after initiation of therapy for the first 3 months, then every 3 months thereafter. Plasma PTH levels should be measured at least every 3 months for 6 months, and every 3 months thereafter. (OPINION)
8A.3 Dosage adjustments for patients receiving active vitamin D sterol therapy should be made as follows:
8A.3a If plasma levels of intact PTH fall below the target range for the CKD stage (Table 15, Guideline 1), hold active vitamin D sterol therapy until plasma levels of intact PTH rise to above the target range, then resume treatment with the dose of active vitamin D sterol reduced by half. If the lowest daily dose of the active vitamin D sterol is being used, reduce to alternate-day dosing. (OPINION)
8A.3b If serum levels of corrected total calcium exceed 9.5 mg/dL (2.37 mmol/L), hold active vitamin D sterol therapy until serum calcium returns to <9.5 mg/dL (2.37 mmol/L), then resume treatment at half the previous dose. If the lowest daily dose of the active vitamin D sterol is being used, reduce to alternate-day dosing. (OPINION)
8A.3c If serum levels of phosphorus rise to >4.6 mg/dL (1.49 mmol/L), hold active vitamin D therapy, initiate or increase dose of phosphate binder until the levels of serum phosphorus fall to =4.6 mg/dL (1.49 mmol/L); then resume the prior dose of active vitamin D sterol. (OPINION)
GUIDELINE 8B. VITAMIN D THERAPY IN PATIENTS ON DIALYSIS (CKD STAGE 5)
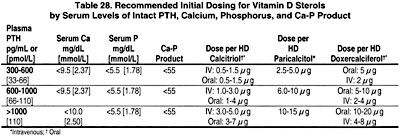
8B.1 Patients treated with hemodialysis or peritoneal dialysis with serum levels of intact PTH levels >300 pg/mL should receive an active vitamin D sterol (such as calcitriol, alfacalcidol, paricalcitol, or doxercalciferol; see Table 28) to reduce the serum levels of PTH to a target range of 150 to 300 pg/mL. (EVIDENCE)
8B.1a The intermittent, intravenous administration of calcitriol is more effective than daily oral calcitriol in lowering serum PTH levels. (EVIDENCE)
8B.1b In patients with corrected serum calcium and/or phosphorus levels above the target range (see Guidelines 3 and 6, respectively), a trial of alternative vitamin D analogs, such as paricalcitol or doxercalciferol may be warranted. (OPINION)
8B.2 When therapy with vitamin D sterols is initiated or the dose is increased, serum levels of calcium and phosphorus should be monitored at least every 2 weeks for 1 month and then monthly thereafter. The plasma PTH should be measured monthly for at least 3 months and then every 3 months once target levels of PTH are achieved. (OPINION)
8B.3 For patients treated with peritoneal dialysis, oral doses of calcitriol (0.5 to 1.0 µg) or doxercalciferol (2.5 to 5.0 µg) can be given 2 or 3 times weekly. Alternatively, a lower dose of calcitriol (0.25 µg) can be administered daily. (OPINION)
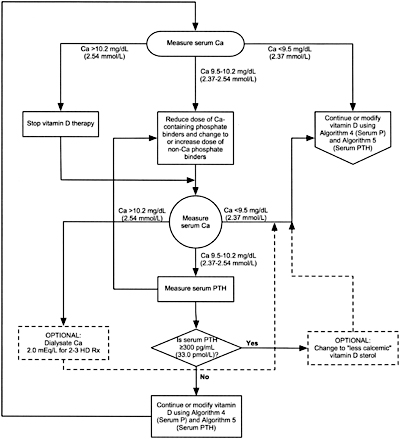
Algorithm 3. Managing Vitamin D sterols based on serum calcium levels.
8B.4 When either hemodialysis or peritoneal dialysis patients are treated with active vitamin D sterols, management should integrate the changes in serum calcium, serum phosphorus, and plasma PTH. Each of these 3 variables is considered separately with suggested interventions based on the various values obtained in Algorithm 3, Algorithm 4, and Algorithm 5. (OPINION)
GUIDELINE 9. DIALYSATE CALCIUM CONCENTRATIONS
9.1 The dialysate calcium concentration in hemodialysis or peritoneal dialysis should be 2.5 mEq/L (1.25 mmol/L). (OPINION)
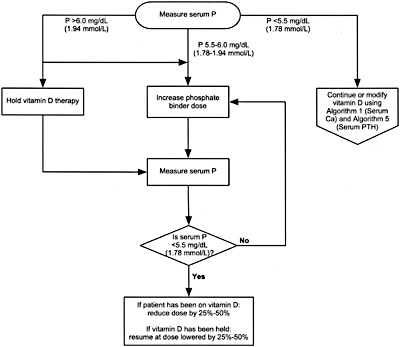
Algorithm 4. Managing Vitamin D sterols based on serum phosphorus levels.
9.2 Higher or lower dialysate calcium levels are indicated in selected patients. (See Clinical Applications.) (OPINION)
GUIDELINE 10. ß2-MICROGLOBULIN AMYLOIDOSIS
10.1 Screening for ß2-microglobulin amyloidosis, including measurement of serum levels of ß2-microglobulin, is not recommended. (OPINION)
10.1a No currently available therapy (except kidney transplantation) can stop disease progression of ß2-microglobulin amyloidosis or provide symptomatic relief. (EVIDENCE)
10.1b Kidney transplant should be considered to stop disease progression or provide symptomatic relief in patients with ß2-microglobulin amyloidosis. (EVIDENCE)
10.1c In patients with evidence of, or at risk for, ß2-microglobulin amyloidosis noncuprophane (EVIDENCE), high-flux dialyzers (OPINION) should be used.
GUIDELINE 11. ALUMINUM OVERLOAD AND TOXICITY IN CKD
11.1 To prevent aluminum toxicity, the regular administration of aluminum should be avoided and the dialysate concentration of aluminum should be maintained at <10 µg/L. (EVIDENCE)
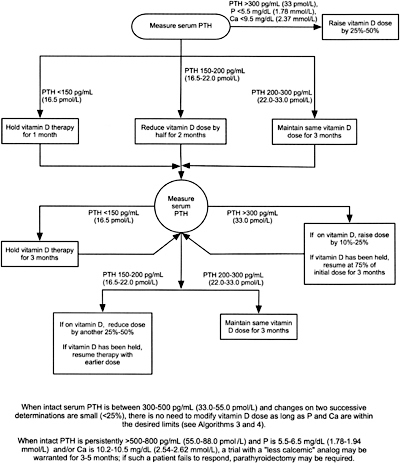
Algorithm 5. Managing Vitamin D sterols based on intact PTH levels.
11.1a CKD patients ingesting aluminum should not receive citrate salts simultaneously. (EVIDENCE)
11.2 To assess aluminum exposure and the risk of aluminum toxicity, serum aluminum levels should be measured at least yearly and every 3 months in those receiving aluminum-containing medications. (OPINION)
11.2a Baseline levels of serum aluminum should be <20 µg/L. (OPINION)
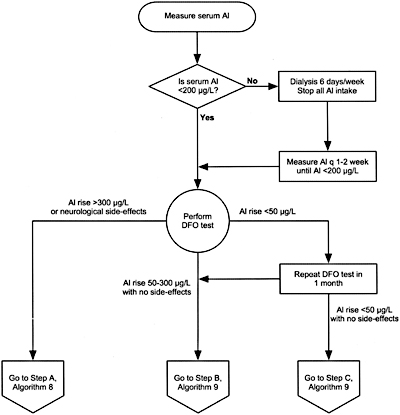
Algorithm 6. Evaluation of aluminum neurotoxicity.
11.3 A deferoxamine (DFO) test should be performed if there are elevated serum aluminum levels (60 to 200 µg/L); clinical signs and symptoms of aluminum toxicity (Table 31), or prior to parathyroid surgery if the patient has had aluminum exposure. (EVIDENCE) (Algorithms 6 and 7)
11.3a The test is done by infusing 5 mg/kg of DFO during the last hour of the dialysis session with a serum aluminum measured before DFO infusion and 2 days later, before the next dialysis session. (OPINION)
11.3b The test is considered positive if the increment of serum aluminum is =50 µg/L. (OPINION)
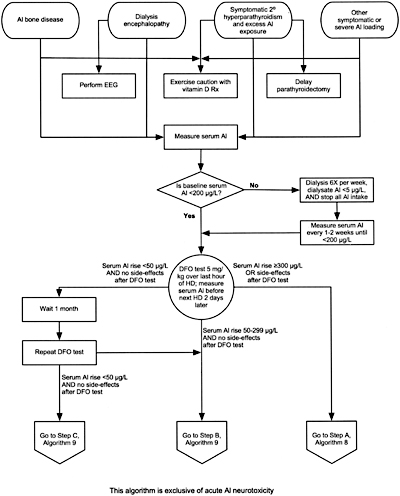
Algorithm 7. Evaluation of aluminum-related disorders:
considerations for DFO test and subsequent DFO treatment.
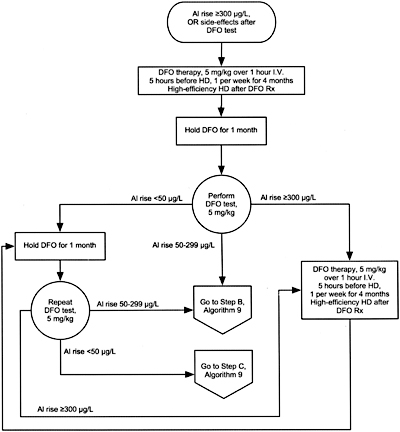
Algorithm 8. DFO treatment after PAl rise =300 µg/L.
11.3c A DFO test should not be performed if the serum levels of aluminum are >200 µg/L to avoid DFO-induced neurotoxicity. (OPINION)
11.4 The presence of aluminum bone disease can be predicted by a rise in serum aluminum of =50 µg/L following DFO challenge combined with plasma levels of intact PTH of <150 pg/mL (16.5 pmol/L). (OPINION) However, the gold standard for the diagnosis of aluminum bone disease is a bone biopsy showing increased aluminum staining of the bone surface (=15% to 25%) using aluminum stain and often adynamic bone or osteomalacia. (EVIDENCE)
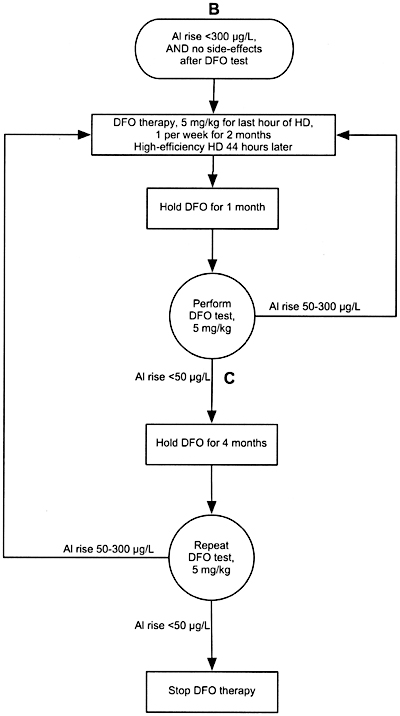
Algorithm 9. DFO treatment after PAl rise between 50 and 300 µg/L.
GUIDELINE 12. TREATMENT OF ALUMINUM AND TOXICITY (ALGORITHM 8 and Algorithm 9)
12.1 In all patients with baseline serum aluminum levels >60 µg/L, a positive DFO test, or clinical symptoms consistent with aluminum toxicity (Guideline 11, Table 31) the source of aluminum should be identified and eliminated. (OPINION)
12.2 In symptomatic patients with serum aluminum levels >60 µg/L but <200 µg/L or a rise in aluminum after DFO >50 µg/L, DFO should be given to treat the aluminum overload. (See Algorithm 8 and Algorithm 9). (OPINION)
12.3 To avoid DFO-induced neurotoxicity in patients with serum aluminum >200 µg/L, DFO should not be given until intensive dialysis (6 days per week) with high-flux dialysis membrane and a dialysate aluminum level of <5 µg/L and until the pre-dialysis serum aluminum level has been reduced to <200 µg/L. (OPINION)
GUIDELINE 13. TREATMENT OF BONE DISEASE IN CKD
The therapeutic approach to bone disease in CKD is based on its specific type. As such, this Guideline encompasses 3 parts: Guideline 13A deals with high-turnover and mixed bone disease, Guideline 13B with osteomalacia, and Guideline 13C with adynamic bone disease.
GUIDELINE 13A. HYPERPARATHYROID (HIGH TURNOVER) AND MIXED (HIGH-TURNOVER WITH MINERALIZATION DEFECT) BONE DISEASE
13A.1 In CKD patients (Stages 3 and 4) who have plasma levels of intact PTH >70 pg/mL (7.7 pmol/L) (Stage 3) or >110 pg/mL (12.1 pmol/L) (Stage 4) on more than 2 consecutive measurements, dietary phosphate intake should be restricted. If this is ineffective in lowering plasma PTH levels, calcitriol (EVIDENCE) or one of its analogs [alfacalcidol (EVIDENCE) or doxercalciferol (OPINION)] should be given to prevent or ameliorate bone disease. (See Guideline 8A.)
13A.2 In CKD patients (Stage 5) who have elevated plasma levels of intact PTH (>300 pg/mL [33.0 pmol/L]), calcitriol (EVIDENCE) or one of its analogs (doxercalciferol, alfacalcidol, or paricalcitol) (OPINION) should be used to reverse the bone features of PTH overactivity (ie, high-turnover bone disease) and to treat defective mineralization. (See Guideline 8B.)
GUIDELINE 13B. OSTEOMALACIA
13B.1 Osteomalacia due to aluminum toxicity should be prevented in dialysis patients by maintaining aluminum concentration in dialysate fluid at <10 µg/L and avoiding the use of aluminum-containing compounds (including sucralfate). (OPINION)
13B.2 Aluminum overload leading to aluminum bone disease should be treated with deferoxamine (DFO). (See Guidelines 11 and 12.) (OPINION)
13B.3 Osteomalacia due to vitamin D2 or D3 deficiency or phosphate depletion, though uncommon, should be treated with vitamin D2 or D3 supplementation (see Guideline 7) and/or phosphate administration, respectively. (OPINION)
13B.3a If osteomalacia due to vitamin D deficiency fails to respond to ergocalciferol or cholecalciferol, particularly in patients with kidney failure (Stage 5), treatment with an active vitamin D sterol may be given. (OPINION) (See Guideline 8B.)
13B.3b Doses of phosphate supplementation should be adjusted upwards until normal serum levels of phosphorus are achieved. (OPINION)
GUIDELINE 13C. ADYNAMIC BONE DISEASE
13C.1 Adynamic bone disease in Stage 5 CKD (as determined either by bone biopsy or intact PTH <100 pg/mL [11.0 pmol/L]) should be treated by allowing plasma levels of intact PTH to rise in order to increase bone turnover. (OPINION)
13C.1a This can be accomplished by decreasing doses of calcium-based phosphate binders and vitamin D or eliminating such therapy. (OPINION)
GUIDELINE 14. PARATHYROIDECTOMY IN PATIENTS WITH CKD
14.1 Parathyroidectomy should be recommended in patients with severe hyperparathyroidism (persistent serum levels of intact PTH >800 pg/mL [88.0 pmol/L]), associated with hypercalcemia and/or hyperphosphatemia that are refractory to medical therapy. (OPINION)
14.2 Effective surgical therapy of severe hyperparathyroidism can be accomplished by subtotal parathyroidectomy or total parathyroidectomy with parathyroid tissue autotransplantation. (EVIDENCE)
14.3 In patients who undergo parathyroidectomy the following should be done:
14.3a The blood level of ionized calcium should be measured every 4 to 6 hours for the first 48 top 72 hours after surgery, and then twice daily until stable. (OPINION)
14.3b If the blood levels of ionized or corrected total calcium fall below normal (<0.9 mmol/L or <3.6 mg/dL corresponding to corrected total calcium of 7.2 mg/dL [1.80 mmol/L]), a calcium gluconate infusion should be initiated at a rate of 1 to 2 mg elemental calcium per kilogram body weight per hour and adjusted to maintain an ionized calcium in the normal range (1.15 to 1.36 mmol/L or 4.6 to 5.4 mg/dL). (OPINION) A 10-mL ampule of 10% calcium gluconate contains 90 mg of elemental calcium.
14.3c The calcium infusion should be gradually reduced when the level of ionized calcium attains the normal range and remains stable. (OPINION)
14.3d When oral intake is possible, the patient should receive calcium carbonate 1 to 2 g 3 times a day, as well as calcitriol of up to 2 µg/day, and these therapies should be adjusted as necessary to maintain the level of ionized calcium in the normal range. (OPINION)
14.3e If the patient was receiving phosphate binders prior to surgery, this therapy may need to be discontinued or reduced as dictated by the levels of serum phosphorus. (OPINION)
14.4 Imaging of parathyroid glands with 99Tc-Sestamibi scan, ultrasound, CT scan, or MRI should be done prior to re-exploration parathyroid surgery. (OPINION)
GUIDELINE 15. METABOLIC ACIDOSIS
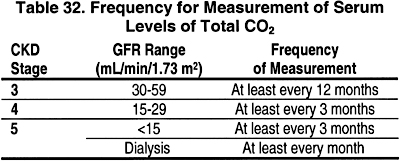
15.1 In CKD Stages 3, 4, and 5, the serum level of total CO2 should be measured.
15.1a The frequency of these measurements should be based on the stage of CKD as shown in Table 32. (OPINION)
15.2 In these patients, serum levels of total CO2 should be maintained at =22 mEq/L (22 mmol/L). (EVIDENCE) If necessary, supplemental alkali salts should be given to achieve this goal. (OPINION)
GUIDELINE 16. BONE DISEASE IN THE KIDNEY TRANSPLANT RECIPIENT
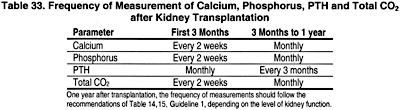
16.1 Serum levels of calcium, phosphorus, total CO2 and plasma intact PTH should be monitored following kidney transplantation. (OPINION)
16.1a The frequency of these measurements should be based on the time following transplantation, as shown in Table 33. (OPINION)
16.2 During the first week after kidney transplantation, serum levels of phosphorus should be measured daily. Kidney transplant recipients who develop persistently low levels of serum phosphate (<2.5 mg/dL [0.81 mmol/L]) should be treated with phosphate supplementation. (OPINION)
16.3 To minimize bone mass loss and osteonecrosis, the immunosuppressive regimen should be adjusted to the lowest effective dose of glucocorticoids. (EVIDENCE)
16.4 Kidney transplant recipients should have bone mineral density (BMD) measured by dual energy X-ray absorptiometry (DEXA) to assess the presence or development of osteoporosis. (OPINION)
16.4a DEXA scans should be obtained at time of transplant and 1 year and 2 years post-transplant. (OPINION)
16.4b If BMD t-score is equal to or less than -2 at the time of the transplant, or at subsequent evaluations, therapy with parenteral amino-bisphosphonates should be considered. (OPINION)
16.5 Treatment of disturbances in bone and mineral metabolism is determined by the level of kidney function in the transplant recipient as provided in Guidelines 1 through 15 for CKD patients. (OPINION)















