NKF KDOQI GUIDELINES
KDOQI Clinical Practice Guidelines for Managing Dyslipidemias in Chronic Kidney Disease
Treating Dyslipidemias
THE APPROACH adopted for adults (Guideline 4) closely parallels that recommended by the ATP III Guidelines,3 and is summarized in Fig 7 and Table 25.
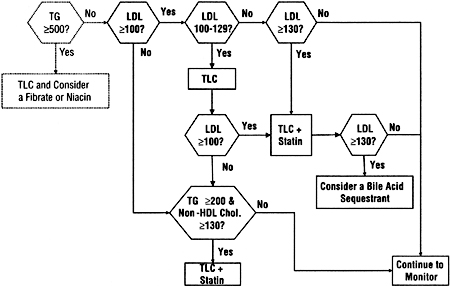
Fig 7. The approach to treatment of dyslipidemias in adults with chronic kidney disease used in these guidelines. Units are in mg/dL. To convert mg/dL to mmol/L, multiply triglycerides by 0.01129 and LDL or non-HDL cholesterol by 0.02586. Abbreviations: TG, triglycerides; TLC, therapeutic lifestyle changes; LDL, low-density lipoprotein; HDL, high-density lipoprotein.
For the rare adult patient with markedly elevated serum triglyceride levels, triglyceride reduction is the principal focus of treatment in order to prevent pancreatitis. Otherwise, high levels of LDL are the focus of treatment. Patients with normal LDL, but high triglycerides, frequently have high levels of remnant lipoproteins. In general, the level of non-HDL cholesterol can be used as a surrogate for increased remnant lipoproteins, and elevated levels of non-HDL cholesterol should be considered for treatment.3 Non-HDL cholesterol is the total cholesterol minus HDL cholesterol (Fig 6).
The approach adopted for adolescents (Guideline 5) is similar to that for adults, but uses higher thresholds for treating LDL and non-HDL cholesterol (Fig 8). These higher thresholds are in deference to the relative lack of evidence for safety and efficacy of treatment in adolescents, and the likelihood that the benefit-to-risk ratio is higher at higher levels of LDL and non-HDL cholesterol.
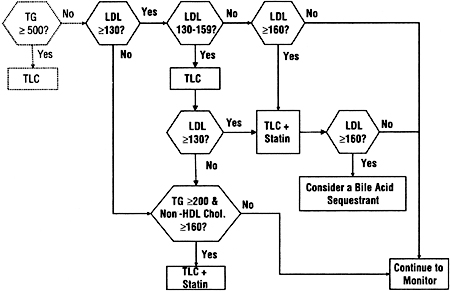
Fig 8. The approach to treatment of dyslipidemias in adolescents with chronic kidney disease used in these guidelines. Units are in mg/dL. To convert mg/dL to mmol/L, multiply triglycerides by 0.01129 and LDL or non-HDL cholesterol by 0.02586. Abbreviations: TG, triglycerides; TLC, therapeutic lifestyle changes; LDL, low-density lipoprotein; HDL, high-density lipoprotein.
Treatment of Adults With Dyslipidemias
Guideline 4
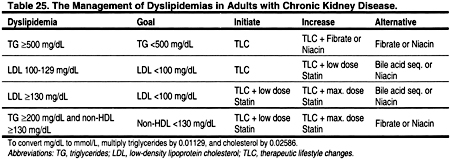
4.1. For adults with Stage 5 CKD and fasting triglycerides ≥500 mg/dL (≥5.65 mmol/L) that cannot be corrected by removing an underlying cause, treatment with therapeutic lifestyle changes (TLC) and a triglyceride-lowering agent should be considered. (C)
4.2. For adults with Stage 5 CKD and LDL ≥100 mg/dL (≥2.59 mmol/L), treatment should be considered to reduce LDL to <100 mg/dL (<2.59 mmol/L). (B)
4.3. For adults with Stage 5 CKD and LDL <100 mg/dL (<2.59 mmol/L), fasting triglycerides ≥200 mg/dL (≥2.26 mmol/L), and non-HDL cholesterol (total cholesterol minus HDL) ≥130 mg/dL (≥3.36 mmol/L), treatment should be considered to reduce non-HDL cholesterol to <130 mg/dL (<3.36 mmol/L). (C)
Rationale for Treating Very High Triglycerides
The general approach to treating dyslipidemia in adults with CKD closely follows the approach adopted by the ATP III (Fig 7 and Table 25). For rare patients with very high triglycerides, treatment of hypertriglyceridemia to reduce the risk for pancreatitis takes precedence over treatment of LDL cholesterol. The ATP III guidelines classify very high fasting triglycerides as ≥500 mg/dL (≥5.65 mmol/L).3 Very high triglycerides are unusual and are generally due to an inherited abnormality in lipoprotein metabolism. For individuals with very high triglycerides, the initial aim of therapy is to prevent acute pancreatitis through triglyceride lowering. Only when triglycerides are <500 mg/dL (<5.65 mmol/L) should attention be focused on LDL cholesterol reduction.
Rarely, severe hypertriglyceridemia can cause pancreatitis in the general population. The incidence of pancreatitis caused by hypertriglyceridemia in patients with CKD is unknown, but it is probably also very low. In 1 observational study, the overall incidence of acute pancreatitis in patients with kidney failure was 2.3% (23/1,001),277 but probably few, if any, of these cases of pancreatitis were caused by dyslipidemias. In another study of 716 patients with kidney failure, 46 (6.4%) were identified as having pancreatitis, while 31 (4.3%) had their first episode of pancreatitis after starting treatment for kidney failure.278 Of 13 patients in whom a first episode of pancreatitis developed after starting hemodialysis, in only 1 was the pancreatitis felt to be caused by hyperlipidemia, and this patient also had cholelithiasis.278 Of 217 patients who received a kidney transplant, pancreatitis developed in 12 (5.5%), but in none of these cases was the pancreatitis believed to be caused by hyperlipidemia. Thus, these limited data suggest that hyperlipidemia is a rare cause of pancreatitis among patients with kidney failure. However, additional studies are needed to better ascertain the incidence of pancreatitis in CKD, and the possible role of dyslipidemias in its pathogenesis.
Treating Very High Triglycerides With Therapeutic Lifestyle Changes
The ATP III guidelines suggest that triglycerides ≥500 mg/dL (≥5.65 mmol/L) should be treated with TLC. In the absence of data on the risk of acute pancreatitis from very high triglycerides in patients with kidney failure, it is reasonable to follow the ATP III guidelines. The ATP III guidelines recommend that TLC include diet, weight reduction, increased physical activity, abstinence from alcohol, and treatment of hyperglycemia (if present). For patients with fasting triglycerides ≥1,000 mg/dL (≥11.29 mmol/L), the ATP III diet recommendations include a very low-fat diet (<15% total calories), medium-chain triglycerides, and fish oils to replace some long-chain triglycerides. Diet should be used judiciously, if at all, in individuals who are malnourished.
Drug Treatment of Very High Triglycerides
If TLC is not sufficient to reduce triglycerides to <500 mg/dL (<5.65 mmol/L), then treatment with a fibrate or nicotinic acid should be considered (Table 25). Studies from the general population suggest that fibrates and nicotinic acid lower triglycerides by 20% to 50% (Fig 9). Statins cause less triglyceride lowering, and bile acid sequestrants may actually increase triglyceride levels.
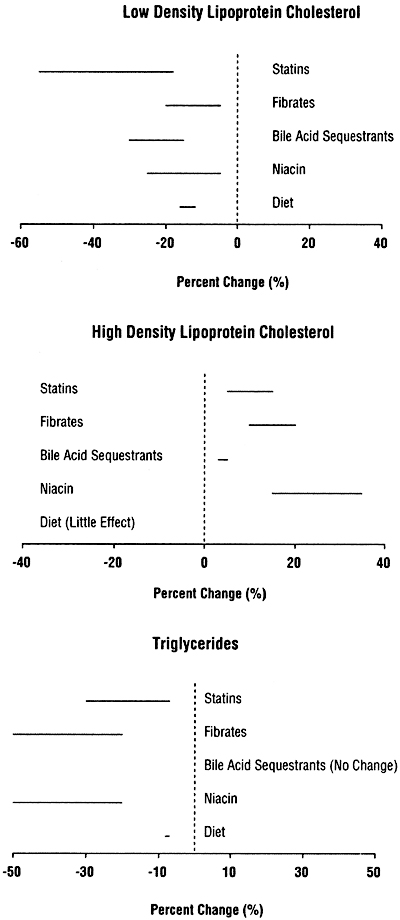
Fig 9. Expected responses to treatment of low-density lipoprotein (upper panel), high-density lipoprotein (middle panel), and triglycerides (lower panel), based on studies in the general population.3
Therefore, when triglycerides continue to be ≥500 mg/dL (≥5.65 mmol/L) despite TLC and/or withdrawal of causative agents, drug treatment should be considered. In general, fibrates are better tolerated than nicotinic acid. In any case, the benefits of drug therapy for hypertriglyceridemia should be weighed against the risks, and the risk of complications (particularly myositis and rhabdomyolysis) is increased in CKD.
Rationale for Treating High LDL Cholesterol
The ATP III Guidelines were developed using rigorous, evidence-based methods. In the absence of data from randomized trials conducted in patients with CKD, it is reasonable to assume that the interventions recommended by the ATP III will similarly reduce ACVD in patients with CKD. However, randomized trials proving that treatment of dyslipidemias reduce the incidence of ACVD ultimately need to be conducted.
The risk of CHD events is markedly increased in patients with CKD.2,4 Therefore, patients with CKD should be considered to have a risk equivalent to that of CHD. This risk category in the ATP III Guidelines includes patients with known ACVD, patients with diabetes, and patients with an expected 10-year risk of CHD >20%. Evidence suggests that patients with CKD have an expected 10-year CHD risk >20%,2,4 thereby justifying their inclusion in this highest risk category.
Treating Proteinuria
Nephrotic-range proteinuria increases total and LDL cholesterol.218-223 In patients with severe proteinuria, triglycerides may also be increased. It may be possible to induce a remission in the nephrotic syndrome by treating the underlying glomerular disease. If not, it may be possible to reduce the level of proteinuria, and thereby improve the patient’s lipid profile. Unfortunately, few randomized controlled trials have documented the lipid-lowering effects of therapies that reduce urine protein excretion, eg, angiotensin II converting enzyme inhibitors, angiotensin II receptor antagonists, and/or low-protein diets.
In a randomized, controlled trial, treatment of 17 nephrotic patients with an angiotensin II converting enzyme inhibitor reduced urine protein excretion from a mean of 5.56 to 4.28 g per day, and decreased mean total cholesterol from 247 to 225 mg/dL (6.39 to 5.82 mmol/L).279 There were no changes in protein excretion or cholesterol levels in 9 placebo-treated controls.279 In a randomized trial of 94 type II diabetic patients with microalbuminuria, treatment with an angiotensin II converting enzyme inhibitor reduced total cholesterol from 245 ± 24 mg/dL (6.34 ± 0.62 mmol/L) to 239 ± 29 mg/dL (6.18 ± 0.75 mmol/L), while cholesterol increased slightly in the placebo group.280 However, in other randomized trials of microalbuminuric type II diabetic patients, angiotensin II converting enzyme inhibitors had little effect on lipid levels.281,282 There are few data on the effects of low-protein diets on lipid levels. In the feasibility phase of the Modification of Diet in Renal Disease study, serum total and LDL cholesterol levels tended to decrease with reduced dietary protein intake.283
There is substantial evidence that angiotensin II converting enzyme inhibitors reduce the rate of kidney disease progression in patients with proteinuria. Therefore, proteinuric patients with CKD should generally be treated with an angiotensin II converting enzyme inhibitor or angiotensin II receptor antagonist, regardless of plasma lipids.2
Treating High LDL With Therapeutic Lifestyle Changes: Diet
There are no randomized trials examining the safety and efficacy of a low-fat, low-cholesterol diet in patients with CKD. However, evidence from the general population suggests that a lipid-lowering diet can reduce LDL.3,30,284 Diet should be used judiciously, if at all, when there is evidence of protein-energy malnutrition. The diet should include <7% of calories as saturated fat, up to 10% of calories as polyunsaturated fat, up to 20% of calories as monounsaturated fat, and a total fat of 25% to 35% of total calories (Table 26 and Appendix 2). The diet should also contain complex carbohydrates (50% to 60% of total calories), and fiber (20-30 g per day). Dietary cholesterol should be <200 mg/day. There are few, if any, adverse effects from this dietary regimen. Some patients with LDL 100-129 mg/dL (2.59-3.34 mmol/L) may achieve the goal of LDL <100 mg/dL (<2.59 mmol/L) with TLC alone.284 Thus, for patients with LDL 100-129 mg/dL (2.59-3.34 mmol/L), it is reasonable to attempt dietary changes for 2-3 months before beginning drug treatment. However, patients with CKD often have a number of other nutritional concerns,285 and it is important to consult a dietitian experienced in the care of patients with CKD.
Treating High LDL With Therapeutic Lifestyle Changes: Exercise and Weight Reduction
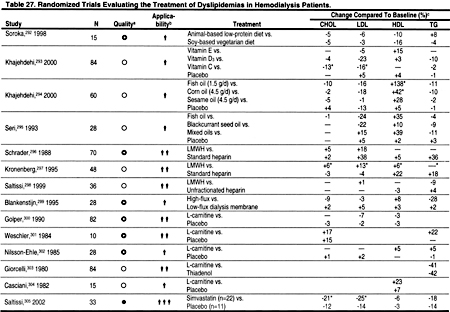
Controlled trials in the general population suggest that exercise training produces small, but significant improvements in dyslipidemias.284,289 Exercise has a number of beneficial effects, independent of those on dyslipidemias, and the lack of adverse effects makes a compelling case for recommending exercise in patients at risk for ACVD.3 At least one small, randomized, controlled trial demonstrated that exercise improved cardiovascular function in hemodialysis patients.290 However, few studies have examined the effects of exercise and/or weight reduction on dyslipidemias in patients with CKD. One randomized, controlled trial examining dyslipidemias in a small number of patients with CKD found that exercise caused a significant decrease only in triglycerides (Table 27).291 Clearly, additional, controlled trials are needed to study the effects of exercise on dyslipidemias and other ACVD risk factors in patients with CKD. Meanwhile, it is recommended that exercise be encouraged in patients with CKD, based on data from studies in the general population.

There are also very few controlled trials examining the effects of weight reduction, with diet and/or exercise, on dyslipidemias in CKD patients. The role of weight reduction, in CKD patients that often have a number of nutritional concerns,285 is unclear. Again, additional studies are needed to define the role of diet, exercise, and weight reduction in dyslipidemic patients with CKD.
Treating High LDL With a Statin
The reduction in LDL that can be achieved with TLC is generally modest. Therefore, TLC alone is usually insufficient to reduce the LDL to the goal of <100 mg/dL (<2.59 mmol/L). In patients who cannot reduce LDL to <100 mg/dL (<2.59 mmol/L) by diet, a statin (3-hydroxy-3-methylglutaryl co-enzyme A reductase inhibitor) should be added, provided that there is no evidence of acute or chronic liver disease. Diet should be continued as an adjunct to the statin. The dose of statin needed to reach the goal of LDL <100 mg/dL (<2.59 mmol/L) varies from patient to patient. Therefore, starting at a low dose and titrating the dose upwards is the best strategy for finding the lowest dose that achieves the goal. This approach will also minimize the frequency and severity of adverse effects. Statins reduced LDL by 18% to 55% in studies in the general population (Fig 9). Statins that are currently approved for use in the United States include atorvastatin, fluvastatin, lovastatin, pravastatin, and simvastatin.
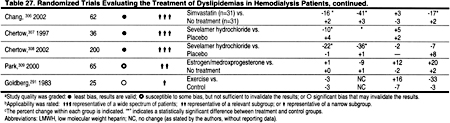
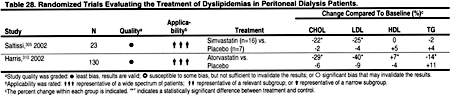
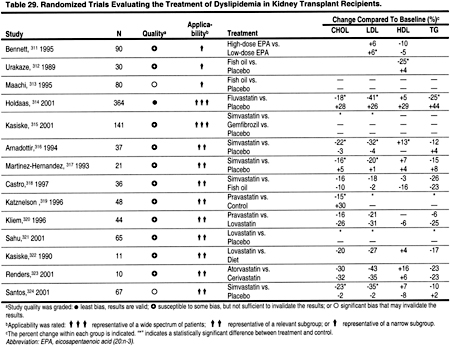
There is strong evidence from studies in the general population that statins reduce CHD events and all-cause mortality. The reduction in mortality and in CHD events is proportional to the reduction in LDL. The literature search identified only 2 small, controlled trials of simvastatin in hemodialysis patients (Table 27), and only 2 randomized trials demonstrating the efficacy of statins in peritoneal dialysis patients (Table 28). There is substantial evidence that statins are safe and effective in reducing LDL in kidney transplant recipients (Table 29). In the absence of strong evidence to the contrary, it is reasonable to assume that statins will reduce LDL and thereby ACVD in most patients with CKD. Statins are clearly the most effective class of anti-lipemic agents for reducing LDL.
Elevated hepatic transaminases occur in 0.5% to 2.0% of patients treated with statins in the general population.325 Therefore, many recommend that baseline alanine and aspartate transferase levels should be obtained, although this is controversial.325 Indeed, whether statins cause hepatotoxicity is controversial. Statins have not been shown to worsen outcomes in patients with chronic transaminase elevations due to hepatitis B or C.325
Patients should also be monitored for signs and symptoms of myopathy. The risk of myopathy from statins is increased by CKD, advanced age, small body frame, and concomitant medications (eg, fibrates, nicotinic acid, cyclosporine, azole antifungals, macrolide antibiotics, protease inhibitors, nefazodone, non-dihydropyridine calcium antagonists, and amiodarone).325 Most experts recommend obtaining a baseline creatinine phosphokinase (CK) level to help in the interpretation of subsequent CK levels. Monitoring statin therapy with routine CK levels is probably not helpful. Patients who develop muscle pain or tenderness should discontinue statin therapy immediately and have a CK level drawn. Elevations greater than 10 times the upper limit of normal are indicative of myositis and require at least temporary cessation of statin therapy.325 For patients with muscle soreness and either normal or mildly elevated CK, levels should be measured weekly, and the patient’s symptoms monitored closely. Often symptoms may improve with a reduction in the dose of the statin. However, if symptoms worsen, the statin should be discontinued. Other causes of myopathy should also be considered, eg, strenuous exercise or hypothyroidism.
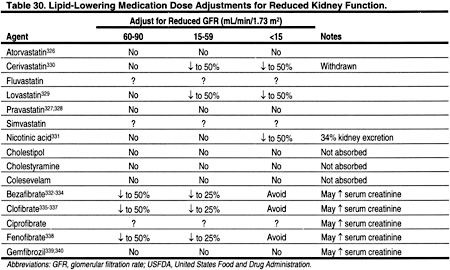
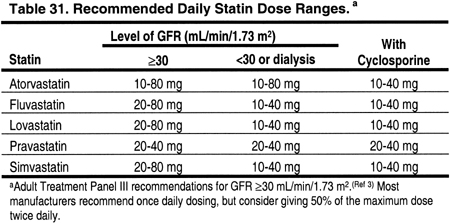
There are limited data on blood levels of statins in patients with CKD (Table 30). In 19 patients with calculated creatinine clearances 13-143 mL/min, the level of kidney function did not affect the blood levels of atorvastatin.326 Pravastatin blood levels were not altered by the level of kidney function in 20 patients with creatinine clearance 15-112 mL/min (0.25-1.87 mL/s),327 or in 12 patients on chronic hemodialysis.328 Lovastatin blood levels were significantly higher in 6 patients with CKD (creatinine clearance 12-39 mL/min [0.20-0.65 mL/s]).329 Therefore, the dose of lovastatin should probably be reduced by 50% in patients with Stages 4 or 5 CKD (GFR <30 mL/min/1.73 m2) (Table 31). The doses of atorvastatin and pravastatin probably do not need to be altered for reduced kidney function per se. Since there are few published data on blood levels for fluvastatin or simvastatin in patients with CKD, we recommend that the doses of these agents be reduced by approximately 50% in patients with Stages 4 or 5 CKD (GFR <30 mL/min/1.73 m2).
Pleiotropic Effects of Statins
Recent data from studies in the general population have indirectly suggested that some of the reduction in ACVD from statins may be independent of their effects on plasma lipids.341,342 Although statins may have favorable effects on endothelial function, coagulation, and plaque stability,343 it has been hypothesized that the effects of statins on systemic inflammation is one of the most important of these pleiotropic effects.343,344 If true, this observation could be important for patients with CKD, who appear to have a high prevalence of elevated C-reactive protein and other markers of systemic inflammation.17-21 On the other hand, analysis of the data from multiple clinical trials in the general population suggests that most, but not all, of the reduction in ACVD from statins can be explained by reductions in LDL.345
The Use of Statins in Patients Receiving Cyclosporine or Tacrolimus
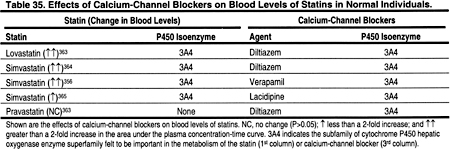
Cyclosporine has been shown to increase the blood levels of virtually every statin that has been investigated (Table 32). The degree that levels are altered may depend on differences in the metabolic pathways of the different statins. The mechanisms for this interaction are not proven, but calcineurin inhibitors may compete with some of the same enzymes responsible for the metabolism of statins. For example, the cytochrome P450 3A4 enzyme, which is thought to be important in the metabolism of lovastatin, simvastatin, and atorvastatin, is inhibited by cyclosporine. Fluvastatin is metabolized through the cytochrome P-450 2C9 pathway. Pravastatin does not rely on the cytochrome P-450 system for metabolism, but is instead metabolized by sulfation. Nevertheless, increased levels of fluvastatin and pravastatin have been reported in patients treated with cyclosporine, although the increases in fluvastatin were not statistically significant (Table 32).
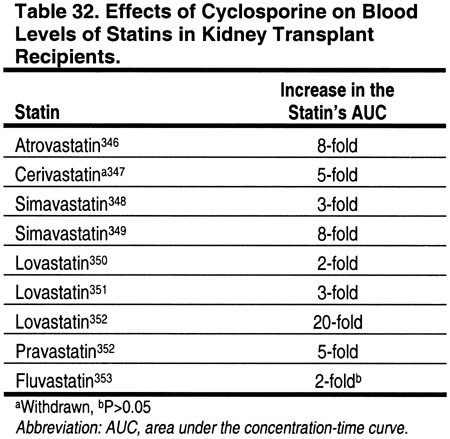
There have been few comparison trials to determine if the increase in statin blood levels from cyclosporine is different for various statins.352 Moreover, the literature search identified only 1 study that examined blood levels of statins in patients treated with tacrolimus. In this study, 4 patients treated with tacrolimus had similar simvastatin blood levels compared to 4 controls who were treated with simvastatin alone.349 However, since the number of patients in this study was very small, and the metabolism of tacrolimus is very similar to that of cyclosporine, it should be assumed (until proven otherwise) that tacrolimus may cause elevations in statin blood levels.
Accumulating evidence suggests that statins can be used safely with cyclosporine if the dose of the statin is reduced (Table 29). It is recommended that the maximum doses of statins be reduced in patients receiving either cyclosporine or tacrolimus (Table 31). The addition of a third agent that is also metabolized by the cytochrome P450 system increases the risk of myositis and rhabdomyolysis, and therefore such combinations should be avoided. The new immunosuppressive agent everolimus had minimal effects on the blood levels of atorvastatin and pravastatin.354 The effects of sirolimus on statins are unknown.
Avoiding Agents That Increase the Blood Levels of Statins
A number of medications may interact with the metabolism of statins and thereby increase statin blood levels. Medications known to increase statin blood levels should either be avoided, or, if necessary, the statin should be reduced or stopped. While this is true for all patients, it is especially true for patients with CKD Stages 4-5, since some statin levels tend to be high in Stage 4-5 CKD patients (Table 30). It is even more critical for interactions to be avoided among kidney transplant patients receiving cyclosporine (and possibly tacrolimus), since cyclosporine often increases statin levels through mechanisms that may be exacerbated by the addition of a third interacting agent.

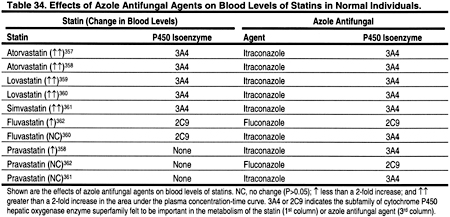
Most medications that are well documented to increase statin blood levels are also metabolized by the hepatic cytochrome P450 enzyme superfamily. These include macrolide antibiotics (Table 33), azole antifungal agents (Table 34), calcium-channel blockers (Table 35), fibrates, and nicotinic acid (Table 36). Other agents that may also increase statin levels include the serotonin re-uptake inhibitors (Table 36), warfarin (Table 36), and grapefruit juice (Table 36).
Adding a Second LDL-Lowering Agent to a Statin
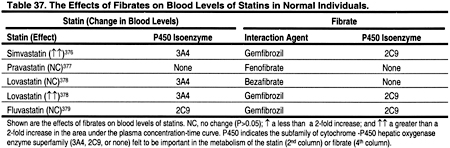
There are very few data on the safety and efficacy of combination therapies in patients with CKD. In general, it is probably wise to avoid the use of a fibrate together with a statin, at least until additional studies are conducted in patients with CKD to establish the safety of this combination. Fibrates lowered LDL by only 5% to 20% in normotriglyceridemic patients in the general population. They may actually increase LDL in patients with high triglycerides. Fibrates may increase the blood levels of statins (Table 37). The mechanisms for the interactions between fibrates and statins are not well understood. It was recently reported that gemfibrozil is a potent inhibitor of the cytochrome P450 2C9 isoform, but had minimal effect on 3A4 in vitro.375
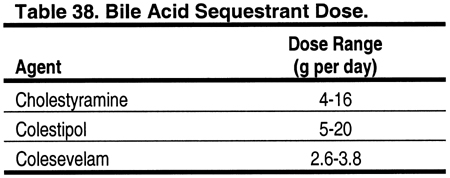
For patients who continue to have LDL ≥100 mg/dL (≥2.59 mmol/L) despite TLC and optimal treatment with a statin, consideration should be given to adding a bile acid sequestrant, if triglycerides are <400 mg/dL (<4.52 mmol/L) (Fig 7 and Table 25). Evidence from studies in the general population indicate that bile acid sequestrants are safe and effective in lowering LDL by 15% to 30% (Fig 9). Bile acid sequestrants can be used in combination with a statin.380 However, there are few studies of the safety and efficacy of bile acid sequestrants in patients with CKD. Cholestyramine, colestipol, and colesevelam hydrochloride are approved for use in the United States (Table 38). Bile acid sequestrants are contraindicated in patients with triglycerides ≥400 mg/dL (≥4.52 mmol/L), since they may increase triglycerides in some patients. They are relatively contraindicated for triglycerides ≥200 mg/dL (≥2.26 mmol/L). It should be noted that the new phosphate-binding agent sevelamer hydrochloride appears to lower lipid levels by mechanisms similar to those of bile acid sequestrants.308

For patients who have triglycerides that preclude the use of a bile acid sequestrant, or for patients who do not tolerate a bile acid sequestrant, nicotinic acid can be considered as an alternative second agent in combination with a statin. Studies in the general population indicate that nicotinic acid reduces LDL by 5% to 25%, reduces triglycerides by 20% to 50%, and raises HDL by 15% to 35% (Fig 9). There are no data on the use of combination therapy with a statin and nicotinic acid in patients with CKD. Adverse effects of nicotinic acid include flushing, hyperglycemia, and hepatotoxicity. Contraindications to nicotinic acid include liver disease, severe gout, and active peptic ulcer disease.
Treating High LDL in Patients Who Cannot Take a Statin
Patients who develop minor adverse effects from a statin may be able to tolerate a reduced dose, or a different statin. However, for patients who do not tolerate a reduced dose or another statin, a second-line agent can be used. Either a bile acid sequestrant or nicotinic acid can be used to effectively reduce LDL cholesterol. For patients who cannot afford the cost of a statin, nicotinic acid offers a cheaper alternative. The phosphate-binding agent sevelamer hydrochloride may also lower total and LDL cholesterol. There have been 2 randomized, controlled trials in CKD patients.307,381 In these studies, sevelamer hydrochloride caused significant reductions in total cholesterol (Table 27).
The Use of Bile Acid Sequestrants in Kidney Transplant Recipients
Bile acid sequestrants may interfere with the absorption of immunosuppressive medications, particularly immunosuppressive agents that bind to lipids. However, some small, uncontrolled studies suggest that a bile acid sequestrant can be used safely, without interfering with the absorption of cyclosporine. In 1 uncontrolled study, co-administration of cholestyramine and cyclosporine in 5 heart transplant recipients did not reduce the area under the concentration-time curve of cyclosporine.382 In another study of 6 kidney transplant patients, administration of cholestyramine 4 hours after a dose of cyclosporine did not reduce the area under the concentration-time curve of cyclosporine.383 Based on these very limited data, it appears that bile acid sequestrants may not have a major effect on cyclosporine absorption. However, it may be prudent to avoid administering a bile acid sequestrant from 1 hour before to 4 hours after the dose of cyclosporine, and to monitor blood levels of cyclosporine.
Unfortunately, there are no published data on the effects of bile acid sequestrants on other immunosuppressive agents. In general, the risks and benefits of adding a bile acid sequestrant to an oral immunosuppression regimen should be carefully weighed. For many patients, the risk of transplant rejection resulting from poor absorption of immunosuppressive medication may outweigh the benefits of a further reduction in LDL from adding a bile acid sequestrant. However, for some patients (eg, patients with severe coronary artery disease), the benefit of a further reduction in LDL may exceed the small risk of adding a bile acid sequestrant.
Similarly, bile acid sequestrants could theoretically interfere with the absorption of statins.374 Therefore, it is probably best to avoid taking the bile acid sequestrant at the same time as any other medication, if this is possible.
Optimizing Immunosuppressive Agents in Kidney Transplant Recipients
For kidney transplant recipients who have LDL ≥100 mg/dL (≥2.59 mmol/L), despite maximal medical management, consideration should be given to changing the immunosuppression protocol to one that is less likely to exacerbate high LDL levels, if this can be done without causing undue risk to the allograft. Options to consider include: (1) tapering and discontinuing prednisone,212,214,255,256 with or without adding or increasing the dose of azathioprine or mycophenolate mofetil; (2) replacing cyclosporine with tacrolimus210,211,215; (3) tapering and discontinuing cyclosporine,215 with or without adding or increasing the dose of azathioprine or mycophenolate mofetil; or (4) discontinuing or replacing sirolimus with an alternative immunosuppressive agent.258,259
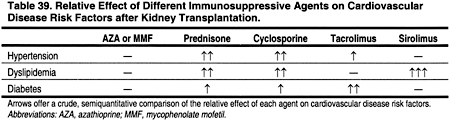
Evidence suggests that discontinuing or replacing prednisone, cyclosporine, or sirolimus may reduce the prevalence and severity of dyslipidemias and other ACVD risk factors such as hypertension and glucose intolerance (Table 23). However, in deciding to change or not to change immunosuppressive agents, the risk of rejection should be weighed against the risk of ACVD. Transplant recipients who are diabetic and/or have known ACVD may have more to gain from changing immunosuppressive agents than patients at lower risk for ACVD. Moreover, the effects of immunosuppression on overall ACVD risk should be taken into account, not just their effects on dyslipidemias (Table 39). For example, different immunosuppressive agents have different effects on blood pressure and post-transplant diabetes, both of which can affect the incidence of ACVD. In any case, the decision to alter immunosuppression should be made only after fully informing the patient of the risks and benefits that are involved.
Rationale for Treating Non-HDL Cholesterol in Patients With High Triglycerides
Non-HDL cholesterol is defined as total cholesterol minus HDL cholesterol. No evidence has directly linked low HDL, high fasting triglycerides, and increased non-HDL cholesterol to ACVD in patients with CKD. However, a growing body of evidence from the general population has suggested that this lipid profile is part of a metabolic syndrome (insulin resistance, obesity, hypertension, and dyslipidemia) that is associated with ACVD.3 Measures that safely and effectively improve this lipid profile should be considered to help reduce the incidence of ACVD in patients with CKD. Indeed, several cross-sectional studies have reported that hemodialysis patients have higher levels of remnant lipoproteins than comparable patients in the general population.104,116-123
Studies in the general population have implicated increased triglycerides as an independent risk factor for ACVD.384,385 It is considered most likely that the risk of high triglycerides is a result of atherogenic, remnant lipoproteins. These include small VLDL and intermediate density lipoproteins (IDL). Since VLDL cholesterol is highly correlated with remnant lipoproteins, VLDL can be combined with LDL cholesterol to enhance risk prediction when triglycerides are high. Non-HDL cholesterol is calculated as total cholesterol minus the HDL cholesterol. In persons with high triglycerides, eg, 200-499 mg/dL (2.26-5.63 mmol/L), most of the cholesterol in non-HDL cholesterol is contained in remnant VLDL. Recent data suggest that non-HDL cholesterol may actually be a better predictor of coronary mortality than LDL.386 Non-HDL cholesterol is also a reasonable surrogate marker for apolipoprotein B, the major apolipoprotein of all atherogenic lipoproteins.387
Studies in the general population suggest that in individuals with triglycerides <200 mg/dL (<2.26 mmol/L) VLDL is not particularly elevated, and non-HDL cholesterol correlates best with LDL cholesterol (Fig 6).387 Therefore, using non-HDL cholesterol as the threshold and target for treatment makes little sense for individuals who do not have high triglycerides. Most clinical trials in the general population have not used non-HDL cholesterol as a target of therapy. Moreover, it is difficult to attribute the risk reduction in these trials to non-HDL cholesterol (compared to VLDL or LDL), because percentage changes in non-HDL cholesterol, VLDL, and LDL closely parallel each other.
Since a normal VLDL cholesterol is usually defined as <30 mg/dL (0.78 mmol/L),388 a reasonable goal for non-HDL cholesterol is one that is 30 mg/dL (0.78 mmol/L) higher than the LDL cholesterol goal of 100 mg/dL (2.59 mmol/L), ie, <130 mg/dL (<3.36 mmol/L).3 The ATP III does not target triglycerides per se for therapy, since triglyceride levels have more day-to-day variability than non-HDL cholesterol, and targeting the latter allows more flexibility in the choice of therapies.3 The ATP III does not target apolipoprotein B for therapy, since (1) standardized measures of apolipoprotein B are not readily available; (2) measures of apolipoprotein B have not been shown to have greater predictability than non-HDL cholesterol in individuals with high triglycerides; and (3) measurement of apolipoprotein B adds to the expense of the usual lipoprotein profile.3
Limited data from studies in hemodialysis patients suggest that, in this population, remnant lipoproteins are elevated even in patients with normal or near-normal triglycerides. In one study, hemodialysis patients showed higher levels of VLDL and IDL, and lower HDL, than age- and sex-matched controls at similar levels of plasma triglycerides.389 This suggests that the triglyceride threshold for treating non-HDL cholesterol in hemodialysis patients should be lower. However, the Work Group concluded that, in the absence of data from randomized trials in hemodialysis patients, it is prudent to use the higher threshold of triglycerides recommended in the ATP-III. Using a triglyceride threshold 200-499 mg/dL (2.26-5.63 mmol/L) for treating non-HDL cholesterol in patients with low LDL means that only patients with very high VLDL and IDL will be treated. Clearly, additional studies are needed to establish whether therapy targeting lower levels of VLDL and IDL is safe and effective in patients with CKD.
Removing Causes of Hypertriglyceridemia and Elevated Non-HDL Cholesterol
Potentially remediable causes of hypertriglyceridemia include obesity, physical inactivity, excessive alcohol intake, high carbohydrate diet, type 2 diabetes, nephrotic syndrome, and some medications such as estrogens and beta-blockers. Corticosteroids are often used in patients with CKD, and corticosteroid withdrawal may decrease plasma cholesterol and triglycerides.212,214,255,256 Similarly, the immunosuppressive agents cyclosporine,210,215 and especially sirolimus,258,259 cause dyslipidemias, and may occasionally cause triglycerides ≥500 mg/dL (≥5.65 mmol/L). For patients who have triglycerides ≥500 mg/dL (≥5.65 mmol/L), consideration should be given to reducing the dose or withdrawing the offending agent. Anabolic steroids can cause dyslipidemia.248-251 The widespread adoption of erythropoietin to treat anemia in patients with CKD has greatly diminished the use of anabolic steroids. However, in some countries, anabolic steroids may still be used when cost precludes the use of erythropoietin. In some cases, the benefits of anemia treatment may outweigh the risks of dyslipidemia induced by anabolic steroids.
Therapeutic Lifestyle Changes for High Triglycerides and Non-HDL Cholesterol
Moderate alcohol consumption (1-2 ounces of alcohol per day) has been linked to a reduced risk for ACVD in the general population. However, excessive alcohol consumption increases the risk for hypertension, dyslipidemias, and ACVD in the general population. There are virtually no studies on the effects of alcohol consumption in patients with CKD.
Studies in the general population have shown that glycemic control with diet, oral hypoglycemic agents, and insulin are effective in raising HDL and lowering fasting triglycerides. However, studies from the general population have produced conflicting results as to whether intensive (versus usual) glycemic control reduces the risk for ACVD.390-392 In addition, patients with diabetes and CKD may be more likely to have adverse effects from intensive glycemic control measures than diabetic patients in the general population. Nevertheless, patients with low HDL and/or high triglycerides should be assessed for diabetes, and diabetic patients with this lipid profile should have as good glycemic control as possible without causing excessive hypoglycemia.
Obesity is also associated with low HDL and/or high triglycerides. Nutritionally sound diets that restrict calories and increased physical activity help to reduce weight in obese patients in the general population. However, there are few studies demonstrating successful weight reduction in obese patients with CKD.
Low-fat diets and increased physical activity have both been shown to raise HDL and reduce triglycerides in the general population. A limited number of studies suggest that these measures may also be effective in patients with CKD.
Dietary fish oil supplements have been shown to reduce triglycerides in studies in the general population. Few studies have examined the effects of fish oil supplements on lipoproteins in patients with CKD, and their results have been inconclusive (Tables 27 and 29).
Drug Therapy for High Triglycerides and Non-HDL Cholesterol
Observational studies in the general population suggest that high triglycerides are independent risk factors for ACVD.384,385 Intervention trials have shown that statins, fibrates, and nicotinic acid reduce the risk of CHD, and indirect evidence suggests that not all of the benefit in these trials is the result of LDL reductions. However, few studies in patients with CKD have examined the relationships between low HDL, high triglycerides, and ACVD, and the results of these studies have been inconclusive (Tables 10, 11, and 12).
Patients who are not already receiving a statin for treatment of LDL, who have fasting triglycerides ≥200 mg/dL (≥2.26 mmol/L), non-HDL cholesterol ≥130 mg/dL (≥3.36 mmol/L), and who do not have liver disease, should be started on a statin along with TLC. In studies in the general population, statins lowered triglycerides by 7% to 30% and increased HDL by 5% to 15% (Fig 9). Furthermore, statins reduced the incidence of major coronary events, CHD mortality, and stroke and all-cause mortality in studies in the general population. Statins are contraindicated in patients with liver disease. A lipid profile and liver enzymes should be obtained within 2-3 months after starting a statin, and 2-3 months following any adjustment in the dose. The Work Group considered whether a statin or a fibrate should be the first-line agent for treatment of non-HDL cholesterol. Although there are compelling theoretical reasons for considering fibrates in this setting, the Work Group concluded that the safety and efficacy of statins for preventing CVD has been more conclusively established in randomized trials in the general population. Clearly, randomized trials examining both statins and fibrates are needed in patients with CKD.
If the statin is tolerated, no further treatment of non-HDL cholesterol is indicated. If the statin is not tolerated at a reduced dose or after switching to another statin, then consider discontinuing the statin and treating instead with a fibrate.
Only 4 randomized, controlled trials of lipid-lowering agents in hemodialysis patients were identified (Table 27). In 1 study of sevelamer hydrochloride, the effects on triglycerides were not statistically significant, and in a small study of simvastatin the effects on triglycerides were reduced compared to placebo (Table 27). Only 2 trials of lipid-lowering agents in peritoneal dialysis patients were identified, and in these studies statins caused a modest reduction in triglycerides (Table 28). Most randomized trials of lipid-lowering agents in kidney transplant patients were with statins, which generally caused a 15% to 25% reduction in triglycerides (Table 29).
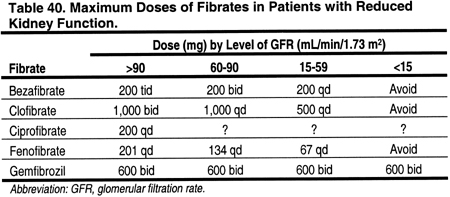
The blood levels of bezafibrate, clofibrate, and fenofibrate are increased in patients with decreased kidney function, compared to controls with normal kidney function (Table 30). In contrast, blood levels of gemfibrozil do not appear to be altered by decreased kidney function (Table 30). Bezafibrate,334,393-401 ciprofibrate,401,402 fenofibrate,401-410 and gemfibrozil410 have been reported to cause increased serum creatinine and blood urea nitrogen levels.334,393,394,399,401,402,404,410 The mechanism for this effect is not known. Since both serum creatinine and blood urea nitrogen are affected, the mechanism presumably involves a reduction in GFR. Indeed, in 1 study, tubular secretion of creatinine was not altered by bezafibrate.395 However, in a study of 13 patients with normal, or mild to moderate kidney disease, fenofibrate increased serum creatinine without altering GFR or plasma flow.408 Gemfibrozil was not thought to cause increased serum creatinine,401,402 but recently there was a report of 2 cases where this occurred.410 Nevertheless, since dose modification for decreased kidney function is not required for gemfibrozil, unlike other fibrates (Table 40), gemfibrozil should probably be considered the fibrate of choice for most CKD patients.
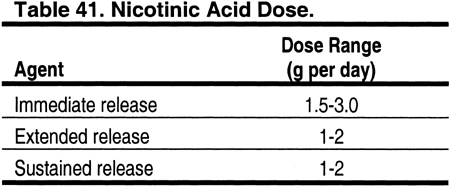
Nicotinic acid can be used in place of fibrates for patients with elevated triglycerides. However, there are almost no data on blood levels of nicotinic acid in patients with CKD. In 1 study, only 34% of a dose of nicotinic acid was excreted in the urine, suggesting that major dose modification may not be necessary in patients with reduced kidney function (Table 41). The incidence of adverse effects from nicotinic acid, eg, flushing and hyperglycemia, is high.411,412 However, there are few studies examining whether the incidence of adverse effects of nicotinic acid is higher in patients with CKD compared to the general population.413 Insulin resistance is common in patients with CKD, and a higher than expected incidence of hyperglycemia from nicotinic acid would not be surprising in CKD patients.
Isolated, Low HDL Cholesterol
Patients with isolated HDL ≥40 mg/dL (≥1.03 mmol/L) should be treated with TLC. However, the pharmacological treatment of isolated low HDL cholesterol is not recommended. There are few data defining the risk of ACVD attributable to isolated, low HDL in the general population or in patients with CKD (Tables 10, 11, and 12). The effects of pharmacological agents on HDL are modest, and the incidence of adverse effects is probably higher in patients with CKD than in the general population. Therefore, the risks of pharmacological therapy to raise HDL (in the absence of high LDL or high triglycerides) probably outweigh the benefits.
Treatment of Adolescents With Dyslipidemias
Guideline 5
5.1. For adolescents with Stage 5 CKD and fasting triglycerides ≥500 mg/dL (≥5.65 mmol/L) that cannot be corrected by removing an underlying cause, treatment with therapeutic lifestyle changes (TLC) should be considered. (C)
5.2. For adolescents with Stage 5 CKD and LDL ≥130 mg/dL (≥3.36 mmol/L), treatment should be considered to reduce LDL to <130 mg/dL (<3.36 mmol/L). (C)
5.3. For adolescents with Stage 5 CKD and LDL <130 mg/dL (<3.36 mmol/L), fasting triglycerides ≥200 mg/dL (≥2.26 mmol/L), and non-HDL cholesterol (total cholesterol minus HDL) ≥160 mg/dL (≥4.14 mmol/L), treatment should be considered to reduce non-HDL cholesterol to <160 mg/dL (<4.14 mmol/L). (C)
Rationale for Treating Very High Triglycerides
Evidence that very high triglycerides can cause pancreatitis in children comes from case reports and small series of patients with familial dyslipidemias.414,415 The incidence of pancreatitis caused by hypertriglyceridemia in adolescents with CKD is unknown. However, it seems prudent to treat very high triglycerides with TLC, if nutrition is otherwise adequate (Fig 8). The safety and efficacy of lowering triglycerides with fibrates and niacin have not been established in adolescents.
Isolated hypertriglyceridemia in adolescents should be treated with TLC. Cases of triglycerides persistently ≥500 mg/dL (≥5.65 mmol/L) are rare, and they are generally due to an inherited metabolic disorder. Drug therapy, eg, low-dose fibrates or nicotinic acid,416 may be warranted. The use of fibrates or nicotinic acid in adolescents has not been well studied417-419; therefore, routine use of these agents cannot be recommended at this time. Patients should be referred, however, to a pediatric lipid specialist for management and to rule out familial hypertriglyceridemia or rare, inherited disorders such as lipoprotein lipase deficiency or apolipoprotein C-II deficiency.420
Rationale for Treating High LDL and High Non-HDL Cholesterol
Atherosclerosis in young adults was first described in 1953.421 Most recently, the PDAY study found that 50% of children 10-14 years old had early fatty streaks, and 8% had fibrous plaques, thus confirming that atherosclerosis begins in childhood.186 Risk factors associated with ACVD in adults are also associated with atherosclerosis in children.186,422 In the Bogalusa Heart Study, body mass index, LDL, and systolic blood pressure were associated with atherosclerotic disease of the aorta and coronary vessels of children.423 Moreover, hypercholesterolemia in children and adolescents persists into adulthood.423 Recent studies of subclinical ACVD in children with familial hypercholesterolemia found an increase in intimal medial thickness of the aorta and carotid arteries compared to that of healthy young children.424 Thus, these and other studies in the general population suggest that ACVD begins in childhood, and that dyslipidemia in children may play an important role in the pathogenesis of ACVD. However, in children with CKD, the relationship between dyslipidemia and subsequent ACVD is unknown.
Approach to Treating High LDL and High Non-HDL Cholesterol
Secondary causes of dyslipidemias should be treated first (Guideline 3). Thereafter, for LDL 130-159 mg/dL (3.36-4.11 mmol/L), TLC should be used first (Fig 8). If, after 6 months of TLC, LDL is ≥130 mg/dL (≥3.36 mmol/L), then consider pharmacological management. If LDL is ≥160 mg/dL (≥4.14 mmol/L), then consider starting atrovastatin at the same time as TLC (Fig 8).
Therapeutic Lifestyle Changes
TLC for children are similar to those recommended for adults (Table 26). Recent studies in the general population have shown that dietary fat restriction is safe in children.425-428 In particular, there have been no adverse effects on growth and development, or nutrition.425-428 However, TLC should be used judiciously, or not at all, in children who are malnourished. If TLC has failed after 6 months, and potential secondary causes of dyslipidemia have been ruled out, drug therapy should be considered.
Drug Therapy
There are few studies examining drug treatment of dyslipidemia in children with CKD. However, a limited number of small, randomized, controlled trials in children and adolescents from the general population have found that statins are safe and effective in lowering LDL.429-432 In particular, statins do not appear to have adverse effects on growth and development.433 A few, very small, uncontrolled trials have likewise reported that statins are safe and effective in patients with nephrotic syndrome.434-436 Thus, although statins are not approved for use in children and adolescents, and additional studies are needed, preliminary data suggest they are safe and effective. Therefore, statins should be considered for therapy in adolescents with CKD and elevated LDL, or in hypertriglyceridemic adolescents with CKD and increased non-HDL cholesterol. Currently, the only statin approved by the United States Food and Drug Administration (USFDA) for use in children and adolescents is atorvastatin.
For adolescents who do not achieve the desired target with a statin, addition of a bile acid sequestrant can be considered (Fig 8). Bile acid sequestrants appear to be safe and effective in improving dyslipidemias in children. Cholestyramine is approved for use in children by the USFDA. Although bile acid resins are safe in children of all ages, adherence to therapy is often poor due to the high incidence of adverse effects.437-439 No dosage adjustment is required in patients with CKD. However, pediatric dosages have not been established. In children 6-12 years of age, doses of anhydrous cholestyramine 80 mg/kg 3 times a day, not to exceed 8 g per day, can be used (Product Information Questran, 2000; Product Information Questran Light, 2000). Adverse effects are common and include constipation, abdominal discomfort, nausea, flatulence, vomiting, diarrhea, heartburn, anorexia, and indigestion. In children and adults treated with cyclosporine, bile acid sequestrants should probably be administered between cyclosporine doses. Bile acid sequestrant powders are generally mixed with 4-6 ounces of fluid, and several glasses of water between doses are recommended. The fluid recommended with bile acid powders may limit their use in dialysis or CKD patients who have been prescribed strict fluid restrictions. The newer bile acid sequestrant colesevelam has not yet been studied in children, and thus cannot be recommended at this time. Similarly, the phosphate-binding (and lipid-lowering) agent sevelamer hydrochloride has not been studied in children.
Bile acid sequestrants can increase triglycerides, and hypertriglyceridemia is common in children with CKD. Bile acid resins are relatively contraindicated in patients with triglycerides ≥200 mg/dL (≥2.26 mmol/L), and definitely contraindicated in patients with triglycerides ≥500 mg/dL (≥5.65 mmol/L). Other potential, long-term adverse effects of bile acid resins include deficiencies of vitamins A, E, and folic acid. In studies with long-term follow-up, a folic acid supplement was required; however, anemia from folate deficiency was not observed.440,441 In CKD patients, hyperhomocysteinemia is more common than in the general population, and therefore the potential for adverse effects from folate deficiency caused by bile acid sequestrants is potentially greater. Taken together, these considerations suggest that bile acid resins should be used with caution in children, and close monitoring for adverse effects such as vitamin deficiencies are warranted.
Currently, atorvastatin is the only USFDA-approved statin for children, and it is approved for post-pubertal males with familial hypercholesterolemia. However, more recent data in boys with familial hypercholesterolemia suggest that lovastatin 10-40 mg can safely decrease LDL by 21% to 36%.442,443 Similar results were reported with pravastatin 5-20 mg.444 Additional data on long-term safety, especially with respect to growth and nutrition, are needed before statins can be recommended for use in children of all ages.




















