2.1 The most accurate diagnostic test for determining the type of bone disease associated with CKD is iliac crest bone biopsy with double tetracycline labeling and bone histomorphometric analysis. (EVIDENCE)
2.2 It is not necessary to perform bone biopsy for most situations in clinical practice. However, a bone biopsy should be considered in patients with kidney failure (Stage 5) who have:
2.2.a Fractures with minimal or no trauma (pathological fractures); (OPINION)
2.2.b Suspected aluminum bone disease, based upon clinical symptoms or history of aluminum exposure; (OPINION) (See Guideline 12)
2.2.c Persistent hypercalcemia with PTH levels between 400-600 pg/mL.
2.3 Bone radiographs are indicated in patients with clinical manifestations suggestive of avascular necrosis (AVN), symptomatic proximal femoral slipped epiphyses (SCFE), rickets, or for the assessment of skeletal maturation. (EVIDENCE)
2.4 Dual-energy X-ray absorptiometry (DXA) should not be used to monitor bone mineral density (BMD) in children with CKD. (OPINION)
The renal bone diseases represent a spectrum of skeletal disorders that range from high-turnover lesions to low-turnover osteodystrophy. Patients may change from one histological subtype to another over time, according to the degree of renal insufficiency and the type of specific treatment of renal osteodystrophy. The use of bone biopsy has contributed substantially to our current understanding of the different subtypes of renal bone diseases. Indeed, quantitative histomorphometry of bone provides information about the status of skeletal mineralization, the structural characteristics of cancellous and cortical bone, the levels of osteoclastic and osteoblastic activities, and the presence of marrow fibrosis.
Bone Biopsy
Determinations of bone formation are estimated by the use of the technique of double tetracycline labeling. Patients are given either demeclocycline or tetracycline HCl; the doses should not exceed 10 mg/kg/day in a tid dosage. Phosphate-binding agents should not be given while patients are receiving the antibiotic. The antibiotics are given on 2 consecutive days, followed by a 10- to 20-day interval when no antibiotic is given and then a second, 2-day course of antibiotics is given; the bone biopsy should be performed within 3-7 days after finishing the second course of antibiotics. In addition to the bone histomorphometry, special staining can be used for identification of aluminum or iron in bone. Iliac crest bone biopsy can be done safely with minimal morbidity.
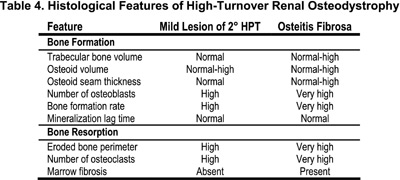
Patients with histological features of high-turnover renal osteodystrophy may have a bone lesion defined as osteitis fibrosa or the mild lesion of 2° HPT. Osteitis fibrosa is the most common high-turnover lesion of renal osteodystrophy in pediatric patients treated with maintenance dialysis. The disorder is characterized by histological evidence of active bone formation with increase in the number and size of osteoclasts and in the number of resorption bays, or Howship's lacunae within cancellous bone (Table 4). Fibrous tissue is found immediately adjacent to bony trabeculae, or it may accumulate more extensively within the marrow space. Osteoblastic activity is increased and the combined increase in osteoblastic and osteoclastic activity accounts for the high rates of bone remodeling and turnover.
The other bone disorder of high-turnover, the mild lesion of 2° HPT, is characterized by only moderate increases in osteoclastic activity and bone formation and without evidence of peritrabecular fibrosis (Table 4). This disorder is a less severe manifestation of hyperparathyroid bone disease. Serum PTH levels are elevated but to a lower degree than in those with osteitis fibrosa, although in some instances it is difficult to assess the degree of fibrosis without a bone biopsy. Tetracycline-based measurements of bone formation are useful for distinguishing this subgroup from those with either normal or reduced rates of bone formation. In order to carefully characterize the different subtypes of renal osteodystrophy according to bone formation rates (BFRs), it is imperative to have tetracycline-based determinations of bone formation in children with normal renal function to be used as controls.
Patients with low-turnover bone renal osteodystrophy may have a bone histological manifestation of osteomalacia or adynamic/aplastic osteodystrophy. The lesion of osteomalacia is characterized by excess osteoid, which accumulates in bone because of a primary defect in mineralization. Osteoid seams are wide and they have multiple lamellae; the extent of trabecular bone surfaces covered with osteoid also is increased. Osteoblastic activity is markedly reduced and bone formation often cannot be measured because of the lack of tetracycline uptake into bone (Table 5). In contrast, bone biopsies from patients with adynamic/aplastic osteodystrophy have normal or reduced amounts of osteoid, no tissue fibrosis, diminished numbers of osteoblasts and osteoclasts, and low or immeasurable rates of bone formation (see Table 1 in Introduction). Patients with aluminum-related osteomalacia or adynamic bone have elevated the bone aluminum content. Currently, the adynamic lesion of renal osteodystrophy that is not related to aluminum, is the most common lesion of renal osteodystrophy in adult patients treated with dialysis. In contrast, 2° HPT remains the predominant lesion in children with CKD. However, a substantial proportion of children developed adynamic bone after intermittent calcitriol therapy. In adults, currently the most common factors involved in the pathogenesis of adynamic bone are:
As measured by conventional histomorphometry, the mineralization lag time reflects the average value for all osteoid seams, and it is often prolonged in adynamic renal osteodystrophy. In contrast, the osteoid maturation time represents the average only for osteoid seams that are undergoing active mineralization, and it is usually normal in the adynamic lesion. The disparity in these values between adynamic bone and osteomalacia is due to differences in the proportion of osteoid seams undergoing active mineralization at any given point in time.
Some patients demonstrate histological features of both osteitis fibrosa and osteomalacia and this combination is defined as the mixed lesion of renal osteodystrophy. Patients may have biochemical evidence of 2° HPT, but other factors such as hypocalcemia and/or hypophosphatemia may account for the defective mineralization. However, most of the described cases have been associated with aluminum toxicity.
Dual-Energy X-ray Absorptiometry (DXA)
The DXA technique is widely accepted as a quantitative measurement for assessing skeletal status in adults. The World Health Organization criteria for the diagnosis of osteoporosis in adults is based on the comparison of a measured BMD result with the average BMD of young adults at the time of peak bone mass (PBM), defined as a T-score31. A T-score ≤2.5 SD below the mean PBM is used for the diagnosis of osteoporosis. While the T-score is a standard component of DXA BMD results, it is clearly inappropriate to assess skeletal health in children through comparison with peak adult bone mass. At present, there are no evidence-based guidelines for classification of bone health in children.
Dual-energy X-ray absorptiometry is a projectional technique in which three-dimensional objects are analyzed as two-dimensional, and bone is presented as the total bone mineral content (BMC) within the projected bone area. It provides an estimate of BMC expressed as grams per anatomical region (e.g., individual vertebrae, whole body, or hip). Dividing the BMC (g) by the projected area of the bone (cm2) then derives “areal BMD” (g/cm2). This BMD is not a measure of true volumetric density (g/cm3) because it provides no information about the depth of bone. Furthermore, because the bone is presented as the combined sum of cortical and trabecular BMC within the projected bone area, it is not possible to assess the distinct structural characteristics of these discrete bone components.
The DXA technique has several limitations that are pronounced in the assessment of children. These can be broadly classified as (a) difficulties in scan acquisition due to limitations in the bone edge detection software in children with low bone mass32; (b) inadequacy of pediatric reference data across varied maturational stages, ethnic groups, and gender groups in healthy children33; and (c) difficulties in the interpretation of DXA results in children with impaired growth, altered body composition, or delayed maturation due to childhood illness. Although varied techniques have been proposed to address these pitfalls, the third limitation remains the greatest challenge in the assessment of childhood osteopenia.34
A significant limitation of DXA is the reliance on measurement of areal rather than volumetric BMD. Bones of larger width and height also tend to be thicker. Since bone thickness is not factored into DXA estimates of BMD, reliance on areal BMD inherently underestimates the bone density of short people. Therefore, a child with smaller bones will appear to have a mineralization disorder (decreased areal BMD). Recent pediatric studies have recognized the importance of short stature in the assessment of DXA-based measures of BMD in chronic childhood disease, including renal disease35, and have adjusted the DXA BMD result for height and/or weight.34,35 Unfortunately, this too is a misleading approach since healthy children of the same height or weight as a chronically ill child will be younger than the ill child. Skeletal maturity and Tanner stage are key determinants of bone mass, and comparison with less mature controls is a flawed solution to the influence of bone size.
Limitations of DXA in Renal Osteodystrophy
Dual-energy X-ray absorptiometry has been used extensively to evaluate renal osteodystrophy. Clearly, since trabecular and cortical bone behave differently in response to increased parathyroid activity (increase and decrease, respectively), and DXA does not allow distinction of the effects of renal osteodystrophy on the two types of bone, the technique is inherently less useful than three-dimensional techniques such as peripheral QCT. The conflicting data on DXA-derived measures of BMD in patients with renal osteodystrophy are consistent with these limitations. Predictably, DXA results have been quite variable with mean BMD values that are higher than, the same as, or lower than control subjects.35-41 These studies have contributed very little to management of individual patients.
Quantitative computed tomography findings in the vertebrae seem to confirm available histomorphometric data in that trabecular BMD was found to be increased in high-turnover disease (+1.6 SD) and decreased in low-turnover disease (-1.2 SD), relative to age-matched controls.42 On the other hand, vertebral BMD was unable to predict the occurrence of fractures nor was there an association between BMD and the time on dialysis. This is not unanticipated, given that increased BMD in high-turnover disease does not equate with improved structural integrity.
In summary, it is clear that integrated measures of BMD—which do not allow distinction between cortical and trabecular bone and provide no information on bone architecture—are limited in their usefulness to differentiate the spectrum of skeletal disorders in renal osteodystrophy.
Utility of Skeletal Radiography
Bony deformities, most commonly involving rapidly growing bones of the extremities, may occur in children with CKD and chronic metabolic acidosis, osteomalacia associated with vitamin D deficiency, or aluminum-associated adynamic bone disease, and in children with severe 2° HPT. However, any bone may be affected, including the skull, the chest, the spine, or the hip. Atraumatic, pathological fractures are seen more commonly in patients with adynamic bone disease, while fractures may occur in children with CKD with deformed bones subjected to the trauma of normal childhood activities.
The radiographic manifestations of the bone disease associated with CKD in children are quite varied, ranging from the many manifestations of severe 2° HPT to those of frank rickets. Radiographic findings from plain film radiography are technique-dependent, with X-ray voltage, film grain quality, and processing methods influencing the ability to diagnose abnormalities. Magnification techniques can increase the sensitivity of finding abnormalities. Cortical bone is over-represented by plain film radiography when compared to cancellous bone. Bone scintigraphy is a sensitive method for finding bone abnormalities in patients with CKD. However, it may be poorly discriminating, as most patients on maintenance dialysis have diffuse bony uptake of the radionuclide tracer. Accumulation at a single site or two may yield information about pathological fractures in advance of their appearance on plain film radiography. Overall, the technique is employed sparingly. Pathological visceral calcifications may be seen by routine radiography; recently, the use of EBCT in a population of adolescents and young adults with CKD detected pathological coronary calcium deposition.
Standards have been developed in children with normal kidney function to correlate chronological age and pubertal status with radiographic appearances of bones of the hand and wrist, giving rise to a “bone age.” Standard deviations of bone age versus chronological age have been calculated for children with normal kidney function from birth through the end of growth (epiphyseal closure). Bone age is often retarded in children with CKD. It remains unclear if the calculated bone age, if reduced substantially below chronological age, is truly as low as that seen radiographically, since the disease process itself alters the radiographic image in addition to producing a true reduction in bony maturation. Standard deviations of bone age versus chronologic age in children with CKD have not been developed to date. However, the presence of normal or advanced bone age is likely correct, and may guide the clinician as to the utility of using GH to treat the failure in linear growth often seen in children with CKD (see Guideline 11).
Despite considerable advances in our understanding of the pathophysiology, prevention, and treatment of osteodystrophy of CKD, an adequate substitute for bone biopsy in establishing the histological type of osteodystrophy has not been yet developed. Quantitative bone histomorphometry with double tetracycline labeling has become the “gold standard” for the diagnosis of metabolic bone disease in CKD patients.
Given the extensive limitations of DXA in children, and in the setting of renal osteodystrophy, there is no current rationale for performing DXA.
An initial determination of bone age, and the presence or absence of the many radiographic abnormalities in the bones of children presenting with CKD, is useful to the clinician in planning the therapeutic approach for the patient. Rickets may not be appreciated clinically, and the extent of severe hyperparathyroid bone disease can be assessed only in the presence of the lesions, since an absence of bony changes by plain-film radiography does not preclude its presence by the more sensitive technique of dynamic bone histomorphometry. Bone age is an important component of determining the utility of growth hormone therapy.
The importance of bone biopsy has been established by many studies, and it is now accepted as the gold standard for the diagnosis of the various types of osteodystrophy in CKD if performed and interpreted using standard techniques. Normal bone histomorphometry should be performed and the results should be reported in accordance with the standard nomenclature suggested by the American Society of Bone and Mineral Research.43
There are no data that support the utility of DXA in children with CKD.
Definitive diagnosis of AVN, SCFE, or rickets requires skeletal radiography. Assessment of skeletal maturation can only be accomplished by determination of a bone age using skeletal radiography.
There are no recent data utilizing bone biopsy to characterize osteodystrophy in the early stages of CKD in children. Skeletal radiography is an insensitive test when used to classify osteodystrophy in CKD.
Considering the invasive nature of bone biopsy, there is a need to investigate whether other markers of bone disease could be developed to replace bone biopsy for the accurate diagnosis of bone disease in pediatric patients with CKD.
Future studies are needed to determine if noninvasive imaging techniques, such as quantitative computed tomography and magnetic resonance imaging, in combination or not with biochemical determinations, are useful in the assessment of trabecular and cortical manifestations of renal osteodystrophy in children.