Renal artery disease (RAD) is a cause of CKD and hypertension, and can be present in patients with other causes of CKD, such as diabetes or hypertensive nephrosclerosis, and CKD in the kidney transplant.
4.1 For patients in whom there is a clinical suspicion of RAD, the clinician should do one or more of the following:
4.1.a Estimate the probability of RAD using a predictive index derived from clinical characteristics (B)
4.1.b Obtain a noninvasive screening test for RAD (A)
4.1.c Refer to a kidney disease or hypertension specialist for evaluation (C).
4.2 Patients found to have hemodynamically significant RAD should be referred to a kidney disease or hypertension specialist for management (C).
Hemodynamically significant RAD may result in decreased perfusion of the kidney with consequent elevation of systemic blood pressure or decreased GFR. Identification of patients with RAD is an important and challenging diagnosis as it represents a potentially reversible form of hypertension and CKD. Additionally, knowledge of the presence of RAD may have an impact upon clinical decision-making in the use or avoidance of certain classes of antihypertensive agents, such as ACE inhibitors and ARBs. Also, consideration should be given to the possible diagnosis of RAD if GFR declines by a large amount soon after the initiation or increase in dosage of ACE inhibitors or ARBs in patients with CKD presumed due to other causes (see Guideline 11).
The optimal treatment of patients with hypertension and RAD remains an elusive goal as there are no RCTs to compare the effect of medical therapy with revascularization (either surgery, renal angioplasty, or angioplasty with stents) on blood pressure control or the preservation of kidney function. Medical therapy is often required either for the short term prior to intervention, or for the long-term management of unstable patients or for those whose blood pressure is easily controlled with preserved GFR. The medical management of hypertension due to RAD is similar to that of hypertension due to other causes of CKD, but three important distinctions exist. First, hypertension in RAD may be more difficult to control and often requires multiple medications from different classes (see Guidelines 8, 9,10). Secondly, following treatment, early decline in kidney function may occur more frequently and may be larger in magnitude (see Guideline 11). Finally, coexistent atherosclerotic carotid and coronary disease are more prevalent and may require specific intervention (see Guideline 7).
Definitions
RAD is defined as a stenosis of the main renal artery or its proximal branches. The majority of RAD in adults is caused by atherosclerosis, but may also be due to fibrous dysplasia of the renal artery. In children, the majority of RAD is caused by fibrous dysplasia.187-191
Significant RAD is defined anatomically if there is a >50% stenosis of the lumen by renal angiography and is usually considered to be hemodynamically significant if the stenosis exceeds 75%. Hemodynamically significant RAD may result in renovascular hypertension (RVHT) or ischemic nephropathy.
RVHT is defined as systemic hypertension due to hemodynamically significant RAD. RVHT can be effectively treated with ACE inhibitors or ARBs, but may be complicated by an increased risk for adverse effects, specifically an acute decline in GFR soon after beginning therapy.
Ischemic nephropathy is defined as decreased GFR due to hemodynamically significant RAD. Ischemic nephropathy can be treated with revascularization of the kidney.
Noninvasive screening tests for RAD include duplex ultrasonography, captopril renography, captopril plasma renin activity (PRA) test, computerized tomographic angiography (CTA), and magnetic resonance angiography (MRA).
Strength of evidence
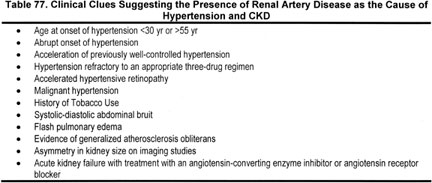
Clinical clues suggestive of RAD (Strong). Traditional clinical clues suggestive that RAD may be the etiology of the CKD or hypertension are shown in Table 77. The most common clinical clues are resistant hypertension or decreased GFR, especially in the setting of generalized atherosclerosis.
Early decline in GFR following ACE inhibitors or ARBs may be a clinical clue suggestive of RAD. The magnitude in the reduction of kidney function after initiation of ACE inhibitors or ARBs is a critical observation. In one study, the average increase in serum creatinine from the baseline value in patients with hemodynamically significant RAD was approximately 40% during a controlled exposure to ACE inhibitor therapy.192 Similarly, in a recent review of 12 RCTs evaluating CKD progression, there was a strong association between an early rise in serum creatinine up to 30% from the baseline value, which stabilized within the first 2 months of therapy, and long-term preservation of kidney function.193 This relationship held for patients with baseline serum creatinine values of >1.4 mg/dL. The authors recommended withdrawal of the ACE inhibitors in CKD only if the rise in creatinine exceeds 30% above baseline within the first 2 months of therapy initiation, or if hyperkalemia develops. Of note, a lesser rise in serum creatinine (<15% to 30%) may be seen with reduction of systemic blood pressure with various classes of agents (including ACE inhibitors and ARBs), which is not indicative of occult RAD.193,194
Based on these studies and the reciprocal relationship between changes in serum creatinine and changes in GFR, the Work Group concluded that a reduction in estimated GFR >30% from baseline following ACE inhibitor or ARB therapy should be considered suggestive of occult RAD. If other causes are not identified, discontinuation of the ACE inhibitor or ARB is recommended (Guideline 11). Lesser reductions in estimated GFR (15% to 30%) after ACE inhibitor or ARB therapy require evaluation, but are not suggestive of RAD, and do not necessitate withdrawal of these antihypertensive agents (Guideline 11), as long-term benefits with preservation of GFR have been well documented in other causes of CKD (Guidelines 8, 9,10).
Predictive indices (Moderately Strong). Some clinical characteristics, plus the patient’s age, gender, BMI, serum creatinine, and serum cholesterol have been incorporated to derive a "clinical prediction rule," based upon a scoring system (Table 78) and predictive index (Fig 32).195 This predictive index was derived in a predominantly Caucasian population in the Netherlands and has not been validated in African-American, Hispanic, or native American hypertensive populations. If a hypertensive patient is deemed to be at moderate or high risk of RAD, based upon the predictive index, the clinician should obtain a noninvasive screening study or refer to a hypertension specialist for evaluation and management.
Fig 32. Use of the score to estimate the probability of RAD. The vertical axis shows the predicted probability of renal artery stenosis in patients with drug-resistant hypertension as a function of the sum score. The sum score was derived from the prediction rule (Table 78). Thin (gray) lines represent 95% confidence intervals. Reproduced with permission.195
Noninvasive screening tests for RAD (Strong). Frequently utilized noninvasive screening tests for RAD include duplex ultrasonography, captopril renography, captopril plasma renin activity (PRA) test, computerized tomographic angiography (CTA), and magnetic resonance angiography (MRA).196-198 A meta-analysis of the results of these noninvasive tests calculated the receiver operator curves (ROC) for each test and concluded that the MRA and CTA were the preferred screening tests for RAD.199 Both MRA and CTA offer the advantage of demonstrating the anatomy of the main renal arteries, although MRA may not allow visualization of the proximal branches in some cases. The disadvantage of CTA is the necessity of a potentially nephrotoxic contrast exposure—especially in patients with compromised kidney function. The MRA may offer an advantage in patients with decreased GFR, in whom the avoidance of potentially toxic contrast is a consideration. Cost-effectiveness was not studied in the meta-analysis, although this may also influence decision-making.
The availability and local expertise with specific technologies may have an impact on selection of the appropriate noninvasive screening test. Duplex ultrasonography may be a viable option to detect RAD in many centers with local expertise with this technology. Duplex ultrasonography has the advantage of providing both anatomical and functional assessment of renal arteries without using potentially nephrotoxic contrast material; however, disadvantages are that it may be unreliable in obese individuals, time-consuming, and highly operator-dependent. Duplex ultrasonography may be predictive of eventual outcome with revascularization. In a study of nearly 6,000 hypertensive patients, a high resistance index ([1 - end diastolic velocity/maximal systolic velocity] × 100%) was predictive of extremely poor outcomes following revascularization with decline in kidney function and inability to reduce blood pressure by more than 10 mm Hg.200 A resistance index below 80 was predictive of success for improvement in blood pressure control with stable or improved kidney function.
Captopril-stimulated renography also has a high specificity and sensitivity for identifying hemodynamically significant RAD in a hypertensive population with unilateral RAD and preserved kidney function. The additional advantage of captopril-stimulated renography is that it avoids exposure to potentially nephrotoxic contrast. The disadvantage of this type of technology is that it provides functional information but does not delineate the anatomy of the vascular bed. Moreover, it loses specificity and sensitivity with decreasing GFR. Its utility has not been well studied in patients with CKD stage 4 or 5.196-199,201-203 Furthermore, the ACE inhibitor renogram offers a possible alternative to assess the clinical significance of a stenotic lesion and predict successful outcome of revascularization.
Referral to specialists (Weak). Detailed discussion of management of RAD is beyond the scope of this guideline. As with other causes of CKD, the goals of therapy for RAD are to lower blood pressure, slow progression of kidney disease, and reduce risk of cardiovascular disease. However, for a number of reasons as indicated below, management is complex, and in the opinion of the Work Group, should be undertaken in consultation with a kidney disease or hypertension specialist.
There is insufficient evidence to suggest a specific target blood pressure in RAD. There are no controlled trials comparing conventional blood pressure targets to a lower blood pressure goal (<130/80 mm Hg) in RAD. Patients with atherosclerotic RAD have a high risk of CVD, and it is reasonable to hypothesize that they may benefit from a blood pressure goal of <130/80 mm Hg, similar to others at high risk of CVD. However, it is uncertain whether a lower target blood pressure would adversely affect kidney perfusion in uncorrected RAD and whether a change in perfusion pressure would have a beneficial or detrimental effect on the progression of kidney disease. Moreover, patients with uncorrected atherosclerotic RAD often have resistant hypertension, and the proportion of patients that can attain a lower blood pressure goal is not known. Under any circumstance, kidney function should be closely monitored in these patients following initiation or change in hypertensive therapy.
There is insufficient evidence to indicate that any class of antihypertensive agents is preferred for slowing kidney disease progression in RAD. Multiple short-term studies have documented the improved efficacy of ACE inhibitors versus other agents in controlling hypertension in patients with RAD, yet reductions in GFR were not uncommon.204-208 Reductions in GFR were more likely to occur in patients with high-grade bilateral RAD or RAD of a solitary kidney. Unfortunately, there have been no large, long-term RCTs in RAD comparing therapy with ACE inhibitors or ARBs versus other antihypertensive agents on blood pressure control, kidney disease progression, or cardiovascular morbidity and mortality.209 A small, nonrandomized study in unilateral RAD patients comparing therapy with versus without ACE inhibitors suggested a survival benefit for patients treated with ACE inhibitors.210
In the clinical setting where RAD is associated with the onset of rapidly progressive heart failure and pulmonary edema ("flash pulmonary edema"), it is reasonable to apply agents that block the renin angiotensin system, beta-blockers, and loop diuretics for treatment of systolic and/or diastolic heart failure and ECF volume overload, as tolerated.
If therapy with an ACE inhibitor or ARB alone is not adequate to control blood pressure, several short-term studies document that the addition of a thiazide diuretic can achieve blood pressure control in the majority of patients.204 If target blood pressure is not achieved with a combination of an ACE inhibitor or ARB and a diuretic, additional antihypertensive agents may be added as outlined in Guideline 7. If an ACE inhibitor or ARB cannot be used, other antihypertensive agents can be used, such as beta-blockers, diuretics, or calcium-channel blockers. In patients with high-grade bilateral RAD, some have postulated that a calcium-channel blocker may be more useful because it may dilate the afferent preglomerular arterioles and thereby maintain GFR.
Side-effects of ACE inhibitors and ARBs may be more frequent in RAD than in other types of CKD. ACE inhibitor or ARB therapy may be considered in RAD, although the monitoring of side-effects including hypotension, hyperkalemia, or sudden decrease in GFR should be more frequent than in other causes of CKD. In hemodynamically significant RAD, hypertension and GFR may be dependent upon angiotensin II (AII). Therapy with an ACE inhibitor or ARB removes the AII-mediated vasoconstriction, particularly at the efferent arteriole, thereby lowering glomerular pressure and GFR—especially in patients with high-grade bilateral or solitary kidney RAD. Hence, an acute deterioration of kidney function (reduction in GFR exceeding 30%) in response to therapy with an ACE inhibitor or ARB may be a clue to occult RAD.
ACE inhibitors and ARBs may reduce the aldosterone-mediated effect on potassium excretion, resulting in hyperkalemia. Patients with a reduced GFR, or those ingesting potassium supplements or potassium-sparing medications, are at the highest risk. Serum potassium should be monitored as indicated in Guideline 11.
There is insufficient evidence to prefer one of the variety of options for revascularization versus medical therapy. The four current therapeutic options available to treat patients with RAD include (1) medical management, (2) surgical revascularization, (3) percutaneous transluminal renal arteriography (PTRA), and (4) stents. The optimal method for managing patients with RAD remains a debatable issue as there are no RCTs addressing the risks and benefits of medical versus surgical versus PTRA versus stents. In the absence of such data, the optimal management of a specific patient with RAD is subject to debate and controversy. The risks and benefits of medical versus interventional therapy are complex. In the opinion of the Work Group, the decision regarding specific management for a patient with RAD should be undertaken in consultation with a kidney disease or hypertension specialist. Decision-making about specific therapy requires clinical expertise to balance the potential benefits versus complications of a planned strategy or intervention. Advantages of interventional therapy for RAD for selected patient populations may include control of hypertension, preservation of kidney function, or reduction in the frequency of flash pulmonary edema. However, potential complications of planned interventions include contrast toxicity, atheroembolic, dissection, and bleeding.
Three randomized trials of medical therapy versus PTRA (without stents) demonstrated a slight benefit in blood pressure control with fewer medications (2.5 versus 3.0) but kidney function was unaffected in patients randomized to PTRA.211,212 A randomized, controlled trial of medical therapy versus PTRA with stenting has not been completed. A recent randomized trial of medical versus surgical revascularization did not demonstrate any difference in composite "stop points," including uncontrolled hypertension, 50% decrease in GFR, cardiovascular event, or mortality.213 Nonetheless, selected patients may derive benefit in blood pressure control and/or kidney function following interventions. A detailed analysis of these intervention studies is beyond the scope of this guideline. In the opinion of the Work Group, the clinician should refer patients with hemodynamically significant RAD to a kidney disease specialist or a hypertension specialist who should attempt to balance the potential advantages and disadvantages of a specific management strategy.
Recommendations for the optimal management of patients with RAD are limited by the absence of long-term, randomized trials comparing medical management versus revascularization interventions upon blood pressure control, GFR, and cardiovascular morbidity and mortality. Similarly, the absence of long-term, randomized trials of the medical management of RAD comparing therapy with or without ACE inhibitors and ARBs precludes recommending ACE inhibitors or ARBs as preferred antihypertensive agents in RAD.
Routine use of prediction equations to estimate the probability of RAD requires (1) availability of equations in easy-to-use formats, such as handheld calculators and (2) education of clinicians about the use of such predictions.
A long-term, RCT should be conducted to compare the effect of medical management versus percutaneous transluminal renal angioplasty (PTRA) with stenting on blood pressure control, progression of kidney disease, and cardiovascular morbidity and mortality. Such a trial should aim to answer the questions of who is the ideal candidate for interventional therapy, and what are the benefits, if any, of an invasive approach to RAD.
The clinical predictive index needs to be validated in a hypertensive population composed of African American, Hispanics, and American Indians as well as Caucasians.